Download Files:
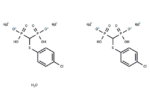
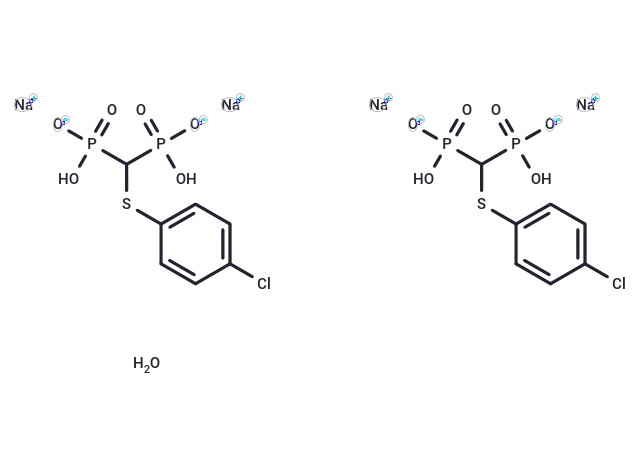
Tiludronate disodium hemihydrate
2,432 CAD
Only 1000 item(s) left in stock.
Products Details
Product Description
– Tiludronate (Tiludronic Acid) disodium hemihydrate is an orally active bisphosphonate compound used primarily in metabolic bone disorder research. It functions as an osteoregulator and acts as a potent inhibitor of the osteoclast vacuolar H+ -ATPase, possessing antiresorptive and anti-inflammatory properties [4].
Web ID
– T39037
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C14H16Cl2Na4O13P4S2
References
– Reginster JY, et al. Prevention of postmenopausal bone loss by tiludronate. Lancet. 1989;2(8678-8679):1469-1471.
CAS Number
– 155453-10-4
Molecular Weight
– C14H16Cl2Na4O13P4S2
SMILES
– OP([O-])(C(P([O-])(O)=O)Sc1ccc(Cl)cc1)=O.OP([O-])(C(P([O-])(O)=O)Sc2ccc(Cl)cc2)=O.[Na+].[Na+].O.[Na+].[Na+]
Pathway
– Others
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
1000 in stock
1000 in stock