Download Files:
69 CAD – 869 CADPrice range: 69 CAD through 869 CAD
Products Details
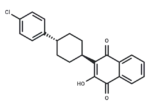
Product Description
Web ID
Storage Temperature
Shipping
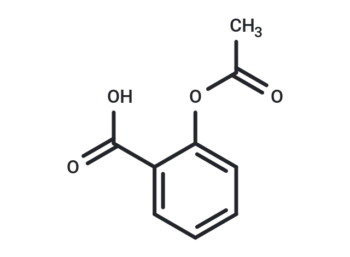
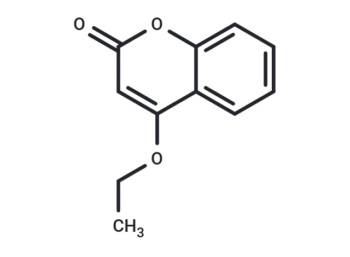
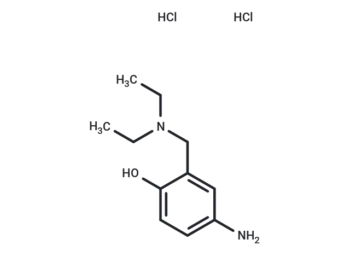
Molecular Formula
References
CAS Number
Molecular Weight
Compound Purity
SMILES
Target
Pathway
Product type
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
1000 in stock
1000 in stock