Download Files:
Zimlovisertib
48 CAD – 1,533 CADPrice range: 48 CAD through 1,533 CAD
Products Details
Product Description
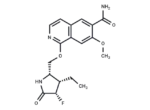
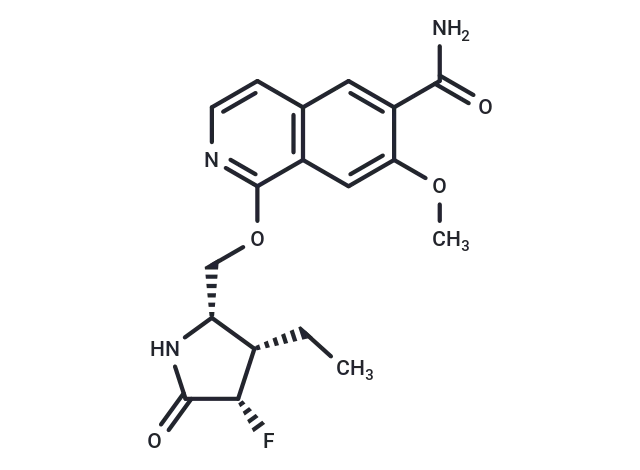
– Zimlovisertib (PF-06650833) is an effective, selective and orally active inhibitor of interleukin-1 receptor-associated kinase 4 (IRAK4) (IC50s: 0.2 and 2.4 nM in the cell and PBMC assay).
Web ID
– TQ0167
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C18H20FN3O4
References
– Lee KL, et al. Discovery of Clinical Candidate 1-{[(2S,3S,4S)-3-Ethyl-4-fluoro-5-oxopyrrolidin-2-yl]methoxy}-7-methoxyisoquinoline-6-carboxamide (PF-06650833), a Potent, Selective Inhibitor of Interleukin-1 Receptor Associated Kinase 4 (IRAK4), by Fragment-Based Drug Design. J Med Chem. 2017 Jul 13;60(13):5521-5542.
CAS Number
– 1817626-54-2
Molecular Weight
– C18H20FN3O4
Compound Purity
– 0.9821
SMILES
– O(C[C@@H]1[C@H](CC)[C@H](F)C(=O)N1)C=2C3=C(C=C(C(N)=O)C(OC)=C3)C=CN2
Pathway
– Immunology/Inflammation|||NF-κB
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
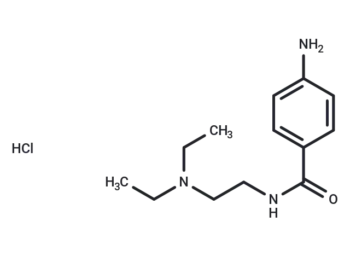
Procainamide hydrochloride
50 CAD – 317 CADPrice range: 50 CAD through 317 CAD
1000 in stock
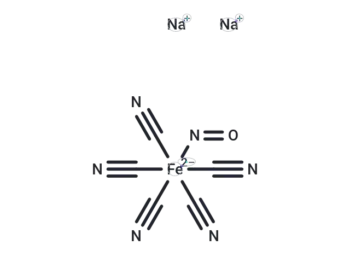
Sodium Nitroprusside
64 CAD – 139 CADPrice range: 64 CAD through 139 CAD
1000 in stock
1000 in stock