Download Files:
Ticagrelor
SKU
HY-10064-10 mg
Category Reference compound
Tags Cardiovascular Disease; Cancer, GPCR/G Protein, P2Y Receptor
$38 – $135
Products Details
Product Description
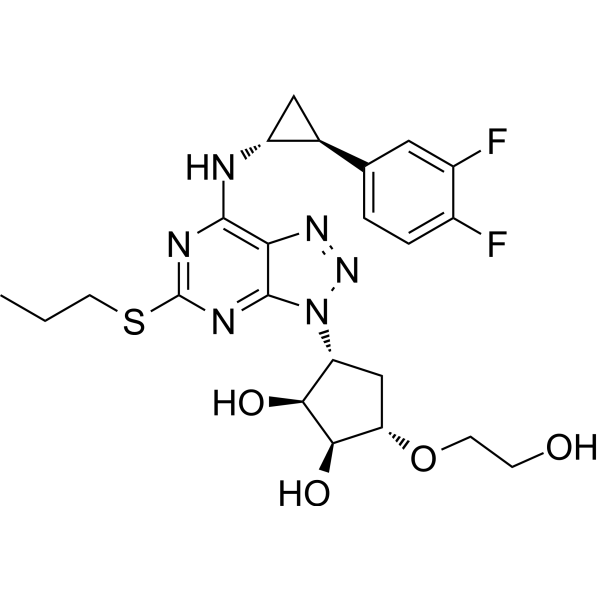
– Ticagrelor (AZD6140) is a reversible oral P2Y12 receptor antagonist for the treatment of platelet aggregation.
Web ID
– HY-10064
Storage Temperature
– -20°C (Powder, protect from light, stored under nitrogen)
Shipping
– Blue Ice
Applications
– COVID-19-immunoregulation
Molecular Formula
– C23H28F2N6O4S
References
– [1]Aungraheeta R, et al. Inverse agonism at the P2Y12 receptor and ENT1 transporter blockade contribute to platelet inhibition by ticagrelor. Blood. 2016 Dec 8;128(23):2717-2728.|[2]Gebremeskel S, et al. The reversible P2Y12 inhibitor ticagrelor inhibits metastasis and improves survival in mouse models of cancer. Int J Cancer. 2015 Jan 1;136(1):234-40.|[3]Sugidachi A, et al. A comparison of the pharmacological profiles of prasugrel and ticagrelor assessed by platelet aggregation, thrombus formation and haemostasis in rats. Br J Pharmacol. 2013 May;169(1):82-9.
CAS Number
– 274693-27-5
Molecular Weight
– 522.57
Compound Purity
– 99.98
SMILES
– O[C@H]1[C@@H](O)[C@H](N2N=NC3=C(N[C@H]4[C@H](C5=CC=C(F)C(F)=C5)C4)N=C(SCCC)N=C32)C[C@@H]1OCCO
Clinical Information
– Launched
Research Area
– Cardiovascular Disease; Cancer
Solubility
– DMSO : ≥ 50 mg/mL
Target
– P2Y Receptor
Isoform
– P2Y12 Receptor
Pathway
– GPCR/G Protein
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






