Download Files:
Products Details
Product Description
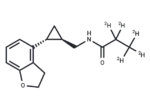
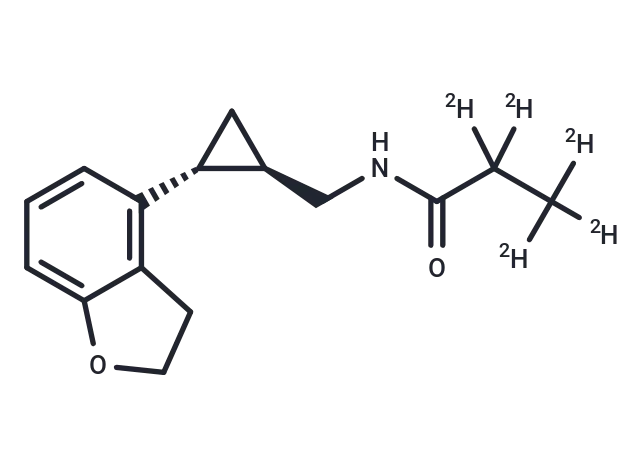
– Tasimelteon-d5 is intended for use as an internal standard for the quantification of tasimelteon by GC- or LC-MS. Tasimelteon is a melatonin (MT) receptor agonist. It selectively binds MT1 and MT2 receptors over a panel of 160 additional receptors and enzymes at 10 µM. Tasimelteon inhibits forskolin-induced cAMP accumulation with EC50 values of 0.79 and 1 nM in NIH3T3 cells expressing the MT1 or MT2 receptor, respectively. Formulations containing tasimelteon have been used in the treatment of non-24-hour sleep-wake disorder.
Web ID
– T70035
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C15H14D5NO2
CAS Number
– 1962124-51-1
Molecular Weight
– C15H14D5NO2
SMILES
– C(NC(C(C([2H])([2H])[2H])([2H])[2H])=O)[C@H]1[C@@H](C1)C2=C3C(=CC=C2)OCC3
Product type
– Isotope products
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
4-Hydroxypropranolol-d7 hydrochloride
365 CAD – 915 CADPrice range: 365 CAD through 915 CAD
1000 in stock