Download Files:
MK-5046
SKU
HY-14342-1 mg
Category Reference compound
Tags Bombesin Receptor, GPCR/G Protein, Metabolic Disease
$100 – $1,650
Products Details
Product Description
– MK-5046 is a potent, selective and orally active Bombesin receptor subtype-3 (BRS-3) allosteric agonist with an IC50 and an EC50 value of 27 and 25 nM for hBRS-3, respectively. MK-5046 inhibits food intake and reduces body weight of diet-induced obese (DIO) mouse models. MK-5046 can be used for the research of obesity[1][2][3].
Web ID
– HY-14342
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Molecular Formula
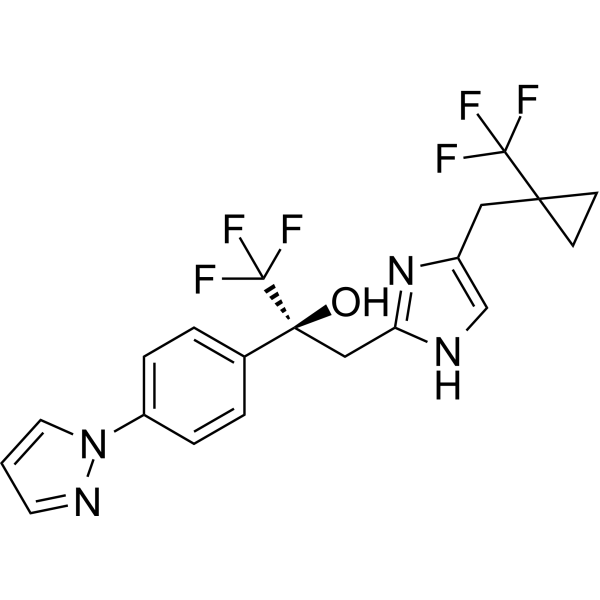
– C20H18F6N4O
References
– [1]Guan XM, et al. Antiobesity effect of MK-5046, a novel bombesin receptor subtype-3 agonist. J Pharmacol Exp Ther. 2011 Feb;336(2):356-364.|[2]Sebhat IK, et al. Discovery of MK-5046, a Potent, Selective Bombesin Receptor Subtype-3 Agonist for the Treatment of Obesity. ACS Med Chem Lett. 2010 Oct 18;2(1):43-47.|[3]Ramos-Alvarez I, et al. The Nonpeptide Agonist MK-5046 Functions As an Allosteric Agonist for the Bombesin Receptor Subtype-3. J Pharmacol Exp Ther. 2022 Aug;382(2):66-78.
CAS Number
– 1022152-70-0
Molecular Weight
– 444.37
Compound Purity
– 99.67
SMILES
– O[C@](CC1=NC(CC2(C(F)(F)F)CC2)=CN1)(C(F)(F)F)C3=CC=C(N4C=CC=N4)C=C3
Clinical Information
– No Development Reported
Research Area
– Metabolic Disease
Solubility
– DMSO : 14.29 mg/mL (ultrasonic)
Target
– Bombesin Receptor
Pathway
– GPCR/G Protein
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






