Download Files:
Linsitinib
53 CAD – 373 CADPrice range: 53 CAD through 373 CAD
Products Details
Product Description
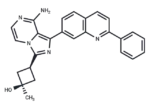
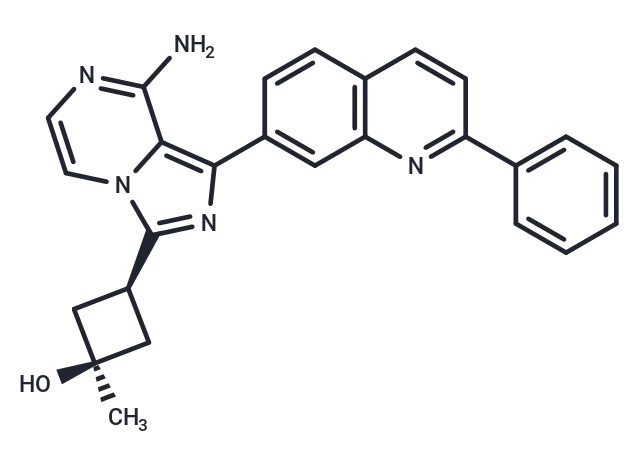
– OSI-906 (Linsitinib (OSI-906)) is an orally bioavailable small molecule inhibitor of the insulin-like growth factor 1 receptor (IGF-1R) with potential antineoplastic activity. Linsitinib selectively inhibits IGF-1R, which may result in the inhibition of tumor cell proliferation and the induction of tumor cell apoptosis. Overexpressed in a variety of human cancers, IGF-1R stimulates cell proliferation, enables oncogenic transformation, and suppresses apoptosis.
Web ID
– T6017
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C26H23N5O
Citations
– 1. Chen F, Shi Q, Pei F, et al. A systems‐level study reveals host‐targeted repurposable drugs against SARS‐CoV‐2 infection. Molecular Systems Biology. 2021, 17(8): e10239
2. Kang J, Guo Z, Zhang H, et al. Dual Inhibition of EGFR and IGF-1R Signaling Leads to Enhanced Antitumor Efficacy against Esophageal Squamous Cancer. International Journal of Molecular Sciences. 2022, 23(18): 10382
3. Yang N, Fan Z, Sun S, et al.Discovery of highly potent and selective KRASG12C degraders by VHL-recruiting PROTACs for the treatment of tumors with KRASG12C-Mutation.European Journal of Medicinal Chemistry.2023: 115857.
4. Guan J, Borenäs M, Xiong J, et al.IGF1R Contributes to Cell Proliferation in ALK-Mutated Neuroblastoma with Preference for Activating the PI3K-AKT Signaling Pathway.Cancers.2023, 15(17): 4252.
5. Alhaddad H, Ospina O E, Khaled M L, et al.Spatial transcriptomics analysis identifies a tumor-promoting function of the meningeal stroma in melanoma leptomeningeal disease.Cell Reports Medicine.2024
References
– Mulvihill MJ, et al. Future Med Chem, 2009, 1(6), 1153-1171.
CAS Number
– 867160-71-2
Molecular Weight
– C26H23N5O
Compound Purity
– 0.9992
SMILES
– NC=1C=2N(C(=NC2C3=CC4=C(C=C3)C=CC(=N4)C5=CC=CC=C5)[C@H]6C[C@@](C)(O)C6)C=CN1
Pathway
– Tyrosine Kinase/Adaptors
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
4-Methoxycoumarine
56 CAD – 266 CADPrice range: 56 CAD through 266 CAD
1000 in stock