Download Files:
50 CAD – 237 CADPrice range: 50 CAD through 237 CAD
Products Details
Product Description
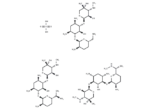
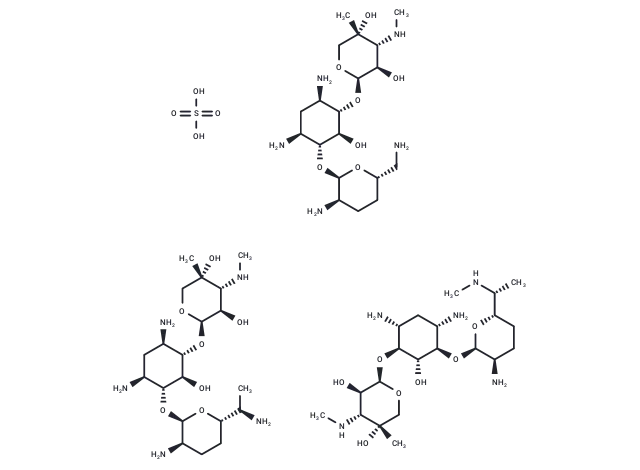
– Gentamicin sulfate (SCH9724) is a wide-spectrum, aminoglycoside antibiotic used to inhibit protein synthesis in sensitive organisms.
Web ID
– T1326
Storage Temperature
– -20℃
Shipping
– Blue Ice
Citations
– 1. Chen M, Xiong H R, Hu Y, et al.Electroacupuncture alleviates sciatic nerve injury and inhibits autophagy in rats.Acupuncture in Medicine.2024: 09645284241280074.
2. Ma X, Li H, Ji J, et al.Overexpression of outer membrane protein A (OmpA) increases aminoglycoside sensitivity in mycobacteria.BMC microbiology.2024, 24(1): 472.
References
– El Mouedden M, et al. Gentamicin-induced apoptosis in renal cell lines and embryonic rat fibroblasts. Toxicol Sci. 2000 Jul;56(1):229-39.
CAS Number
– 1405-41-0
SMILES
– OS(O)(=O)=O.CN[C@@H]1[C@@H](O)[C@@H](O[C@H]2[C@H](N)C[C@H](N)[C@@H](O[C@H]3O[C@H](CN)CC[C@H]3N)[C@@H]2O)OC[C@]1(C)O.CN[C@@H]1[C@@H](O)[C@@H](O[C@H]2[C@H](N)C[C@H](N)[C@@H](O[C@H]3O[C@@H](CC[C@H]3N)[C@@H](C)N)[C@@H]2O)OC[C@]1(C)O.CN[C@H](C)[C@@H]1CC[C@@H](N)[C@@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3OC[C@](C)(O)[C@H](NC)[C@H]3O)[C@H]2O)O1
Target
– Others
Pathway
– DNA Damage/DNA Repair|||Microbiology/Virology|||Metabolism|||Cell Cycle/Checkpoint
Product type
– Natural Product
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock