Download Files:
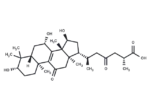
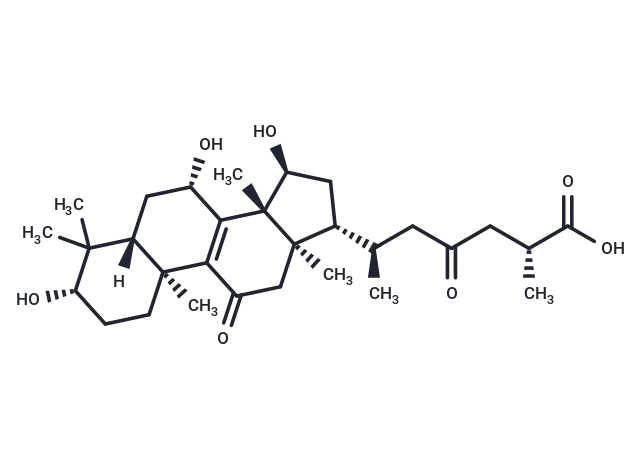
Ganoderic acid C2
88 CAD – 365 CADPrice range: 88 CAD through 365 CAD
Products Details
Product Description
– Ganoderic acid C2 has anti-inflammatory,and anti-tumor-promoting activities. Ganoderic acid C2 can inhibit histamine release, it also has inhibitory effects on the induction of Epstein-Barr Virus early antigen. Ganoderic acid C2 exhibits high inhibitory activity against the rat lens aldose reductase (IC50 = 3.8 µM).
Web ID
– TN1661
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C30H46O7
References
– Guo XY, et al. Structural characterization of minor metabolites and pharmacokinetics of ganoderic acid C2 in rat plasma by HPLC coupled with electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2013 Mar 5;75:64-73.
CAS Number
– 103773-62-2
Molecular Weight
– C30H46O7
Compound Purity
– 0.997
SMILES
– [H][C@@]12C[C@H](O)C3=C(C(=O)C[C@]4(C)[C@H](C[C@H](O)[C@@]34C)[C@H](C)CC(=O)C[C@@H](C)C(O)=O)[C@@]1(C)CC[C@H](O)C2(C)C
Pathway
– Endocrinology/Hormones|||GPCR/G Protein|||Neuroscience|||Metabolism|||Immunology/Inflammation
Product type
– Natural Product
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.