Download Files:
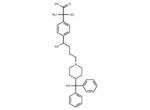
Fexofenadine
48 CAD – 627 CADPrice range: 48 CAD through 627 CAD
Products Details
Product Description
– Fexofenadine (Carboxyterfenadine), an antihistamine pharmaceutical drug, is used to treat allergy symptoms, such as nasal congestion, hay fever, and urticaria. Compared to first-generation antihistamines, Fexofenadine is less able to pass the blood-brain barrier and cause sedation.
Web ID
– T21390
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C32H39NO4
Citations
– 1. Yan F, Gao F. An overview of potential inhibitors targeting non-structural proteins 3 (PLpro and Mac1) and 5 (3CLpro/Mpro) of SARS-CoV-2. Computational and Structural Biotechnology Journal. 2021, 19: 4868.
References
– Miura M, Uno T. Clinical pharmacokinetics of fexofenadine enantiomers. Expert Opin Drug Metab Toxicol. 2010 Jan;6(1):69-74.
CAS Number
– 83799-24-0
Molecular Weight
– C32H39NO4
Compound Purity
– 0.9987
SMILES
– CC(C)(C(O)=O)c1ccc(cc1)C(O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1
Pathway
– GPCR/G Protein|||Immunology/Inflammation|||Neuroscience
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
1000 in stock