Download Files:
Ebrotidine
59 CAD – 155 CADPrice range: 59 CAD through 155 CAD
Products Details
Product Description
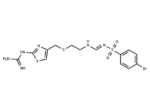
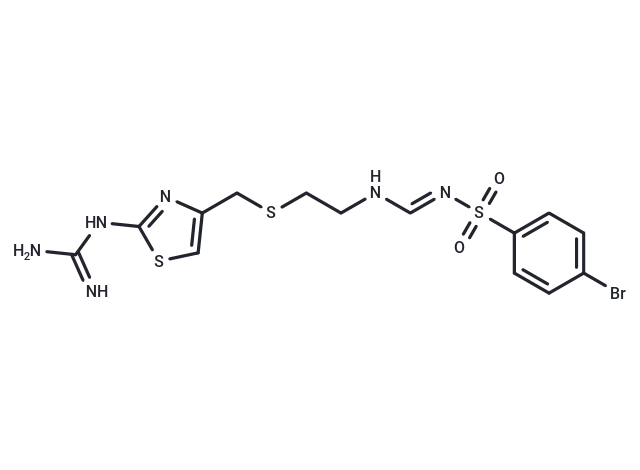
– Ebrotidine (FI3542) is a competitive H2-receptor antagonist with Ki of 127.5 nM. Ebrotidine has a potent antisecretory activity and evidenced gastroprotection.
Web ID
– T15195
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C14H17BrN6O2S3
References
– Agut J, Sánchez JC, Sacristán A, Action of ebrotidine, ranitidine and cimetidine on the specific binding to histamine H1- and H2-receptors. Arzneimittelforschung. 1997 Apr;47(4A):447-9.
CAS Number
– 100981-43-9
Molecular Weight
– C14H17BrN6O2S3
Compound Purity
– 0.975
SMILES
– NC(=N)Nc1nc(CSCCNC=NS(=O)(=O)c2ccc(Br)cc2)cs1
Target
– Apoptosis|||p38 MAPK|||Aromatase
Pathway
– GPCR/G Protein|||Immunology/Inflammation|||Neuroscience
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
4-Hydroxy-6-methylcoumarin
46 CAD – 78 CADPrice range: 46 CAD through 78 CAD
1000 in stock