Download Files:
75 CAD
Only 1000 item(s) left in stock.
Products Details
Product Description
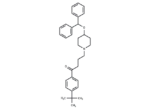
– Ebastine (Kestine) (trade names Evastin, Kestine, Ebastel, Aleva) is a non-sedating H1 antihistamine. It does not penetrate the blood-brain barrier and thus allows an effective block of the H1 receptor in peripheral tissue without a central side effect, i. e not causing sedation or drowsiness.
Web ID
– T2335
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C32H39NO2
References
– Ko CM, et al. J Pharmacol Exp Ther, 1997, 281(1), 233-244.
CAS Number
– 90729-43-4
Molecular Weight
– C32H39NO2
Compound Purity
– 0.9993
SMILES
– C(OC1CCN(CCCC(=O)C2=CC=C(C(C)(C)C)C=C2)CC1)(C3=CC=CC=C3)C4=CC=CC=C4
Pathway
– Neuroscience|||Immunology/Inflammation|||GPCR/G Protein
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
Atenolol Hydrochloride
2,432 CAD – 4,000 CADPrice range: 2,432 CAD through 4,000 CAD
1000 in stock