Download Files:
(E/Z)-Necrosulfonamide
53 CAD – 493 CADPrice range: 53 CAD through 493 CAD
Products Details
Product Description
– (E/Z)-Necrosulfonamide is a novel inhibitor of MLKL.
Web ID
– T7129
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
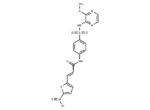
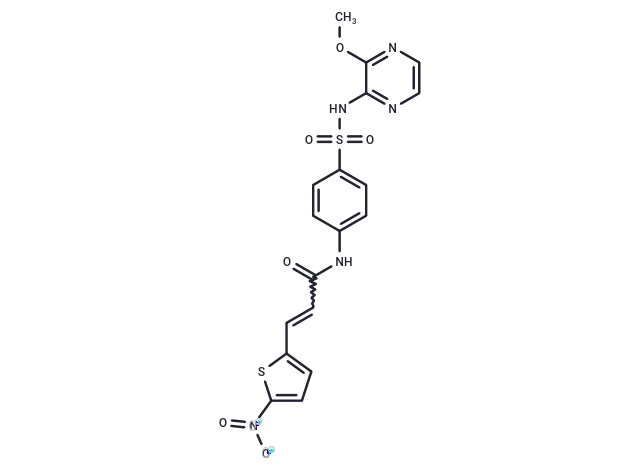
– C18H15N5O6S2
Citations
– 1. Ouyang S, Li H, Lou L, et al. Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biology. 2022: 102317
2. Yi Y, Gao K, Zhang L, et al. Zearalenone Induces MLKL-Dependent Necroptosis in Goat Endometrial Stromal Cells via the Calcium Overload/ROS Pathway. International Journal of Molecular Sciences. 2022, 23(17): 10170.
3. Sun Y, He L, Wang T, et al. Activation of p62-Keap1-Nrf2 Pathway Protects 6-Hydroxydopamine-Induced Ferroptosis in Dopaminergic Cells. Molecular Neurobiology. 2020, 57(11): 4628-4641.
4. Yi Y, Gao K, Lin P, et al. Staphylococcus aureus-Induced Necroptosis Promotes Mitochondrial Damage in Goat Endometrial Epithelial Cells. Animals. 2022, 12(17): 2218.
5. Bi G, Liang J, Shan G, et al.Retinol saturase mediates retinoid metabolism to impair a ferroptosis defense system in cancer cells.Cancer Research.2023: CAN-22-3977.
6. Li Y, Hu G, Huang F, et al.MAT1A Suppression by the CTBP1/HDAC1/HDAC2 Transcriptional Complex Induces Immune Escape and Reduces Ferroptosis in Hepatocellular Carcinoma.Laboratory Investigation.2023, 103(8): 100180.
7. Chen C, Cheng Y, Lei H, et al.SHP2 potentiates anti-PD-1 effectiveness through intervening cell pyroptosis resistance in triple-negative breast cancer.Biomedicine & Pharmacotherapy.2023, 168: 115797.
8. Bi G, Liang J, Bian Y, et al.Polyamine-mediated ferroptosis amplification acts as a targetable vulnerability in cancer.Nature Communications.2024, 15(1): 2461.
References
– Wang Y , Wang J , Wang H , et al. Necrosulfonamide Attenuates the Spinal Cord Injury Via Necroptosis Inhibition[J]. World Neurosurgery, 2018:S1878875018306569.
CAS Number
– 432531-71-0
Molecular Weight
– C18H15N5O6S2
Compound Purity
– 0.9762
SMILES
– COc1nccnc1NS(=O)(=O)c1ccc(NC(=O)C=Cc2ccc(s2)[N+]([O-])=O)cc1
Pathway
– MAPK
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock