Download Files:
Citalopram
48 CAD – 245 CADPrice range: 48 CAD through 245 CAD
Products Details
Product Description
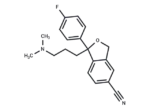
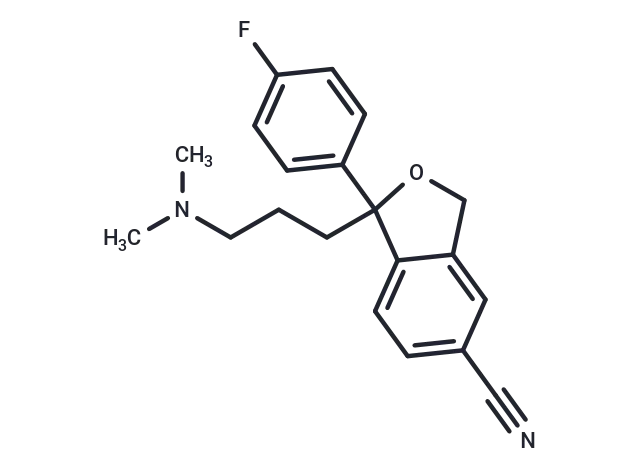
– Citalopram (Lu 10-171 is an orally active, selective serotonin reuptake inhibitor (SSRI), a selective 5-hydroxytryptamine reuptake inhibitor, and a racemic mixture of the S(+)-enantiomer (Escitalopram) and the R(-)-enantiomer.Citalopram exhibits antidepressant activity and enhances serotonergic neurotransmission. It can be used to study Alzheimer’s disease.
Web ID
– T20828
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C20H21FN2O
References
– Carlsson B, et al. Enantioselective analysis of citalopram and escitalopram in postmortem blood together with genotyping for CYP2D6 and CYP2C19. J Anal Toxicol. 2009;33(2):65-76.
CAS Number
– 59729-33-8
Molecular Weight
– C20H21FN2O
Compound Purity
– 0.9989
SMILES
– CN(C)CCCC1(OCc2cc(ccc12)C#N)c1ccc(F)cc1
Pathway
– GPCR/G Protein|||Neuroscience
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
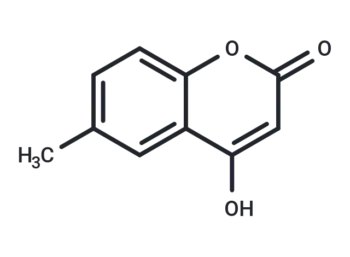
4-Hydroxy-6-methylcoumarin
46 CAD – 78 CADPrice range: 46 CAD through 78 CAD
1000 in stock