Download Files:
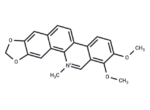
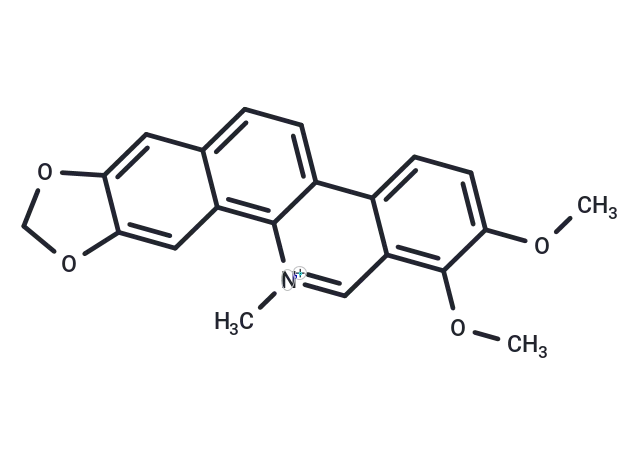
Chelerythrine
80 CAD – 522 CADPrice range: 80 CAD through 522 CAD
Products Details
Product Description
– 1. Chelerythrine (Broussonpapyrine) may have antimanic effect . 2. Chelerythrine can inhibit telomerase activity. 3. Chelerythrine is a well-known protein kinase C inhibitor . 4. Chelerythrine has potential antiproliferative and antitumor effects.
Web ID
– T6S0052
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C21H18NO4
Citations
– 1. Qin X, Liu B, Gao F, et al. Gluconolactone Alleviates Myocardial Ischemia/Reperfusion Injury and Arrhythmias via Activating PKCε/Extracellular Signal-Regulated Kinase Signaling. Frontiers in Physiology. 2022: 455.
2. Cho O, Lee J W, Kim H S, et al.Chelerythrine, a novel small molecule targeting IL-2, inhibits melanoma progression by blocking the interaction between IL-2 and its receptor.Life Sciences.2023: 121559.
References
– Kumar S , Acharya A . Chelerythrine induces reactive oxygen species-dependent mitochondrial apoptotic pathway in a murine T cell lymphoma[J]. Tumor Biology, 2013, 35(1):129-140.
CAS Number
– 34316-15-9
Molecular Weight
– C21H18NO4
Compound Purity
– 0.9886
SMILES
– C[N+]=1C=2C(C=3C(C1)=C(OC)C(OC)=CC3)=CC=C4C2C=C5C(=C4)OCO5
Pathway
– Chromatin/Epigenetic|||Cytoskeletal Signaling|||Apoptosis|||Autophagy
Product type
– Natural Product
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock