Download Files:
2,912 CAD – 4,960 CADPrice range: 2,912 CAD through 4,960 CAD
Products Details
Product Description
Web ID
Storage Temperature
Shipping
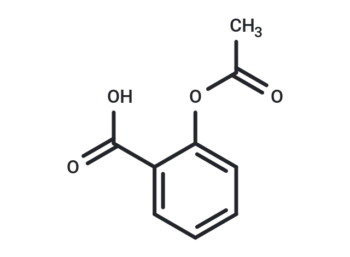
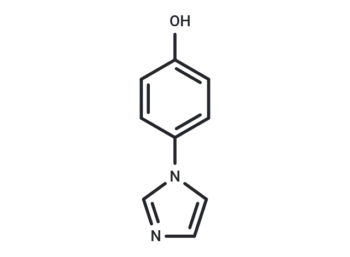
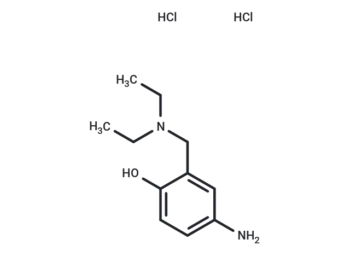
Molecular Formula
References
CAS Number
Molecular Weight
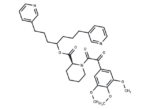
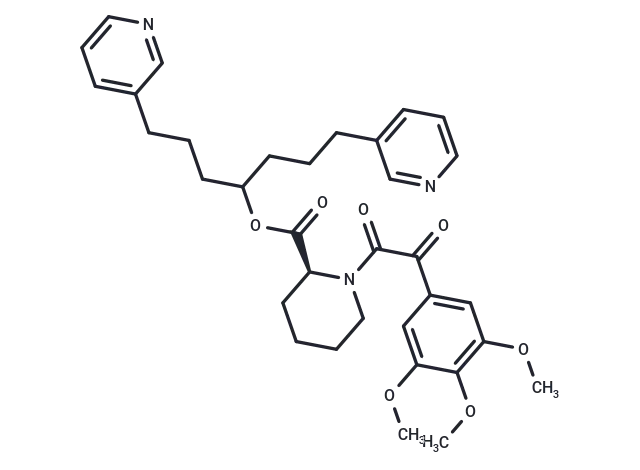
SMILES
Target
Pathway
Product type
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
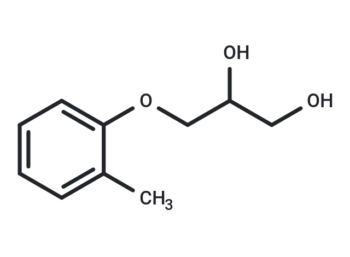
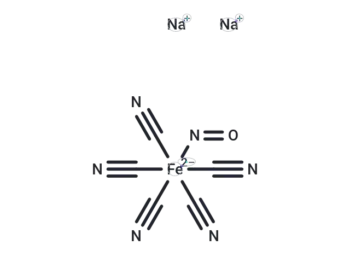
Related Products
1000 in stock
1000 in stock
1000 in stock
1000 in stock