Download Files:
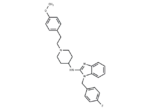
Astemizole
67 CAD – 150 CADPrice range: 67 CAD through 150 CAD
Products Details
Product Description
– Astemizole (Laridal) is a synthetic piperidinyl-benzimidazol derivative with antiallergic properties, acts as a reversible competitive inhibitor of histamine H1 receptors, with less anticholinergic effects compared to related agents. It is a long-acting, non-sedative antihistaminic used in the treatment of seasonal allergic rhinitis, asthma, allergic conjunctivitis, and chronic idiopathic urticaria.
Web ID
– T1278
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C28H31FN4O
Citations
– 1. Wang D, Guo Q, Wu Z, et al.Molecular mechanism of antihistamines recognition and regulation of the histamine H1 receptor.Nature Communications.2024, 15(1): 84.
2. Sun X, Wang Y, Yuan F, et al.Gut symbiont-derived sphingosine modulates vector competence in Aedes mosquitoes.Nature Communications.2024, 15(1): 8221.
3. Molecular mechanism of antihistamines recognition and regulation of the histamine H1 receptor
References
– Salata JJ, et al. Circ Res. 1995 Jan;76(1):110-9.
CAS Number
– 68844-77-9
Molecular Weight
– C28H31FN4O
Compound Purity
– 0.9958
SMILES
– C(N1C=2C(N=C1NC3CCN(CCC4=CC=C(OC)C=C4)CC3)=CC=CC2)C5=CC=C(F)C=C5
Target
– Others
Pathway
– Neuroscience|||GPCR/G Protein|||Immunology/Inflammation|||Membrane transporter/Ion channel
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
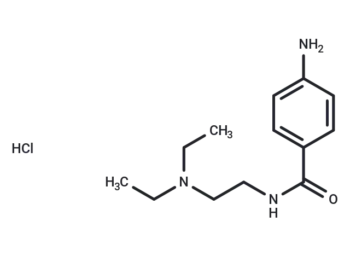
Procainamide hydrochloride
50 CAD – 317 CADPrice range: 50 CAD through 317 CAD
1000 in stock
1000 in stock
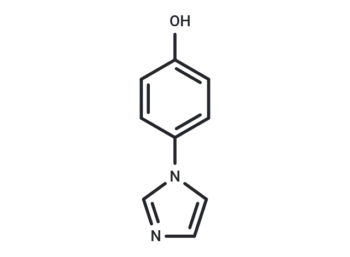
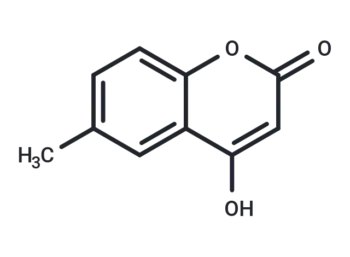
4-Hydroxy-6-methylcoumarin
46 CAD – 78 CADPrice range: 46 CAD through 78 CAD
1000 in stock