Download Files:
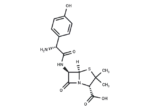
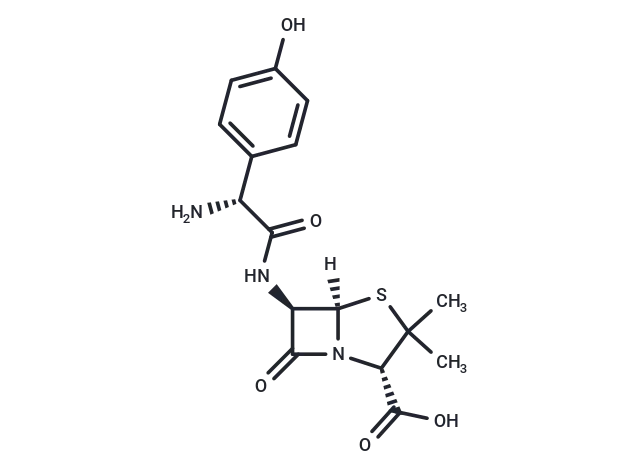
Amoxicillin
66 CAD – 77 CADPrice range: 66 CAD through 77 CAD
Products Details
Product Description
– Amoxicillin (Amoxycillin) binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. Amoxicillin Anhydrous is the anhydrous form of a broad-spectrum, semisynthetic aminopenicillin antibiotic with bactericidal activity. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This interrupts bacterial cell wall synthesis and results in the weakening of the bacterial cell wall and causes cell lysis.
Web ID
– T1005
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C16H19N3O5S
Citations
– 1. Shen X, Zhang W, Peng C, et al. In vitro anti‐bacterial activity and network pharmacology analysis of Sanguisorba officinalis L. against Helicobacter pylori infection. Chinese medicine. 2021, 16(1): 1-19.
2. Yan J, Peng C, Chen P, et al. In-vitro anti-Helicobacter pylori activity and preliminary mechanism of action of Canarium album raeusch. Fruit extracts. Journal of Ethnopharmacology. 2021: 114578.
3. Peng C, Sang S, Shen X, et al. In vitro anti-Helicobacter pylori activity of Syzygium aromaticum and the preliminary mechanism of action. Journal of Ethnopharmacology. 2022: 114995.
References
– Yawalkar N, et al. J Invest Dermatol, 2000, 115(4), 647-652.
CAS Number
– 26787-78-0
Molecular Weight
– C16H19N3O5S
Compound Purity
– 0.9992
SMILES
– [H][C@]12SC(C)(C)[C@@H](N1C(=O)[C@H]2NC(=O)[C@H](N)c1ccc(O)cc1)C(O)=O
Target
– Others
Pathway
– Microbiology/Virology
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
Sodium Nitroprusside
64 CAD – 139 CADPrice range: 64 CAD through 139 CAD
1000 in stock
1000 in stock
1000 in stock