Download Files:
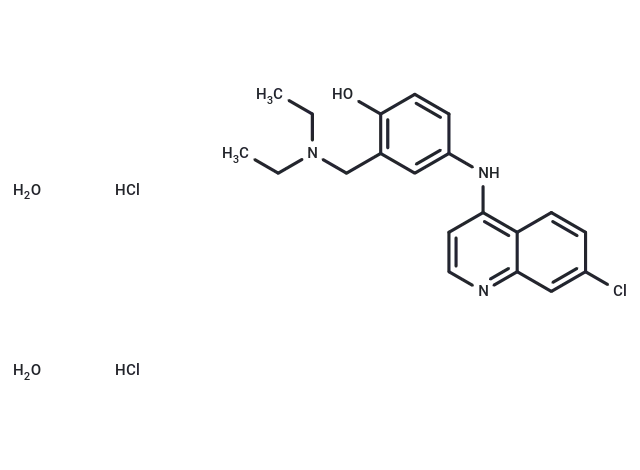
Amodiaquine dihydrochloride dihydrate
53 CAD – 118 CADPrice range: 53 CAD through 118 CAD
Products Details
Product Description
– Amodiaquine dihydrochloride dihydrate (Amodiaquine hydrochloride) is the hydrochloride salt of amodiaquine, an orally active 4-aminoquinoline derivative with antimalarial and anti-inflammatory properties.
Web ID
– T0381
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C20H28Cl3N3O3
References
– Yokoyama A, et al. Eur J Pharmacol. 2007, 558(1-3):179-84.
CAS Number
– 6398-98-7
Molecular Weight
– C20H28Cl3N3O3
Compound Purity
– 0.9997
SMILES
– O.Cl.CCN(CC)Cc1c(O)ccc(Nc2c3ccc(Cl)cc3ncc2)c1.O.Cl
Target
– 5-HT Receptor
Pathway
– Immunology/Inflammation|||Chromatin/Epigenetic|||Microbiology/Virology|||GPCR/G Protein|||Neuroscience
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
1000 in stock