Download Files:
Abemaciclib methanesulfonate
48 CAD – 251 CADPrice range: 48 CAD through 251 CAD
Products Details
Product Description
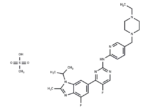
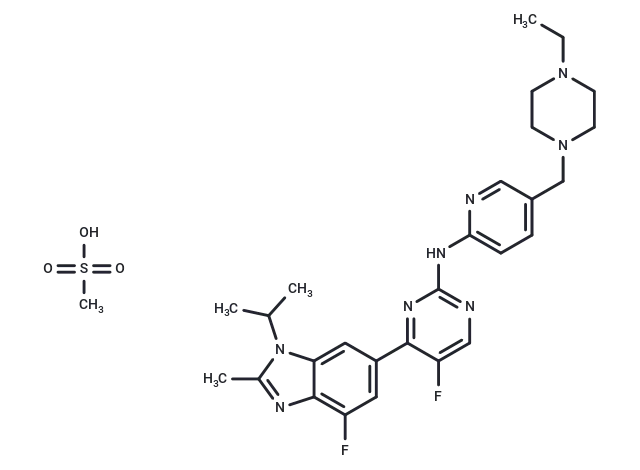
– Abemaciclib methanesulfonate (LY2835219) is a specific and effective inhibitor of CDK4(IC50=2 nM) and CDK6(IC50=10 nM).
Web ID
– T3111
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C27H32F2N8·CH4O3S
Citations
– 1. Li Q, Jiang B, Guo J, et al. INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discovery. 2022, 12(2): 356-371.
2. Ou J, Li H, Qiu P, et al. CDK9 modulates circadian clock by attenuating REV-ERBα activity. Biochemical and Biophysical Research Communications. 2019 Jun 11;513(4):967-973
References
– Sanchez-Martinez et al. Mol Cancer Ther, 2011,10(11 Suppl), Abstract nr B234.
CAS Number
– 1231930-82-7
Molecular Weight
– C27H32F2N8·CH4O3S
Compound Purity
– 0.9944
SMILES
– CS(O)(=O)=O.CCN1CCN(Cc2ccc(Nc3ncc(F)c(n3)-c3cc(F)c4nc(C)n(C(C)C)c4c3)nc2)CC1
Pathway
– Cell Cycle/Checkpoint
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
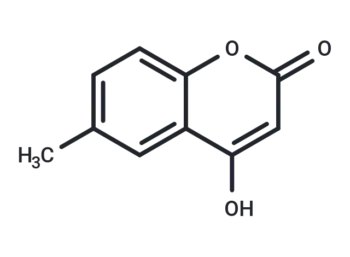
4-Hydroxy-6-methylcoumarin
46 CAD – 78 CADPrice range: 46 CAD through 78 CAD
1000 in stock