Download Files:
2,288 CAD – 5,104 CADPrice range: 2,288 CAD through 5,104 CAD
Products Details
Product Description
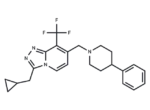
– JNJ-46281222 is a metabotropic glutamate (mGlu) 2-selective, highly potent positive allosteric modulator (PAM) with nanomolar affinity (Kd = 1.7 nM) and high modulatory potency (pEC50 = 7.71).
Web ID
– T15618
Storage Temperature
– -20℃
Shipping
– Blue Ice
Molecular Formula
– C23H25F3N4
References
– Doornbos ML,et al. Molecular mechanism of positive allosteric modulation of the metabotropic glutamate receptor 2 by JNJ-46281222.Br J Pharmacol. 2016 Feb;173(3):588-600.
CAS Number
– 1254980-38-5
Molecular Weight
– C23H25F3N4
SMILES
– FC(F)(F)c1c(CN2CCC(CC2)c2ccccc2)ccn2c(CC3CC3)nnc12
Target
– Phosphatase
Pathway
– Neuroscience
Product type
– Small Compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock
1000 in stock
1000 in stock