Download Files:
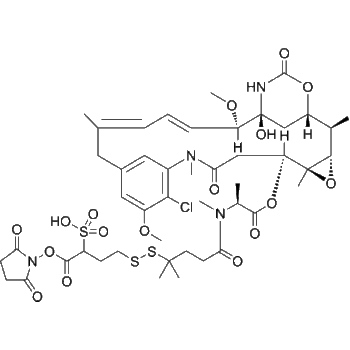
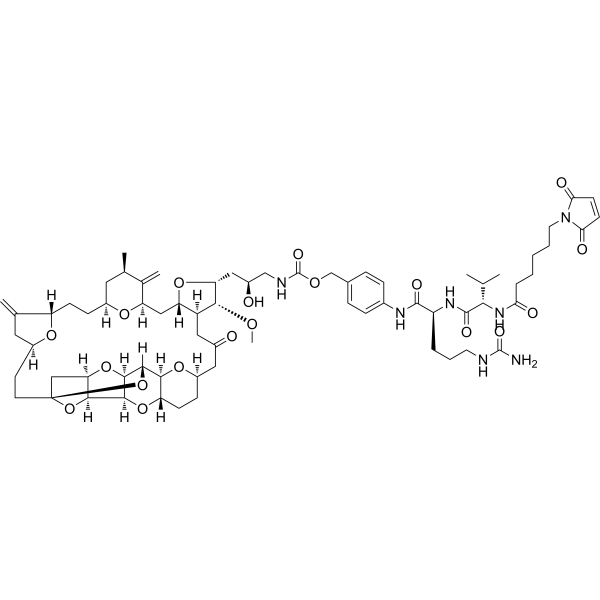
Mal-(CH2)5-Val-Cit-PAB-Eribulin
SKU
HY-139642-10 mg
Category ADC Related
Tags Antibody-drug Conjugate/ADC Related, Cancer, Drug-Linker Conjugates for ADC
$980 – $8,000
Products Details
Product Description
– Mal-(CH2)5-Val-Cit-PAB-Eribulin is a agent-linker conjugate for ADC with potent antitumor activity by using the anti-microtubule agent, Eribulin, linked via linker Mal-(CH2)5-Val-Cit-PAB[1].
Web ID
– HY-139642
Storage Temperature
– 4°C (Powder, protect from light, stored under nitrogen)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C69H97N7O19
References
– [1]Earl F Albone, et al. Eribulin-based antibody-drug conjugates and methods of use. Patent US20170252458A1.|[2]Watanabe K, et, al. Low-dose eribulin reduces lung metastasis of osteosarcoma in vitro and in vivo. Oncotarget. 2019 Jan 4; 10(2): 161-174.
CAS Number
– 2130869-21-3
Molecular Weight
– 1328.54
Compound Purity
– 99.82
SMILES
– C=C1C[C@](O[C@@]1([H])CC[C@](C[C@H]2C)([H])O[C@](C2=C)([H])C[C@@](O[C@@H]3C[C@H](O)CNC(OCC(C=C4)=CC=C4NC([C@H](CCCNC(N)=O)NC([C@H](C(C)C)NC(CCCCCN(C5=O)C(C=C5)=O)=O)=O)=O)=O)([H])[C@]([C@H]3OC)([H])CC6=O)([H])CC[C@@]7(C[C@@]8([H])O9)O[C@]([C@](O[C@](C6)([H])CC%10)([H])[C@@]%10([H])O%11)([H])[C@@]9([H])[C@]%11([H])[C@@]8([H])O7
Clinical Information
– No Development Reported
Research Area
– Cancer
Solubility
– DMSO : 100 mg/mL (ultrasonic)
Target
– Drug-Linker Conjugates for ADC
Pathway
– Antibody-drug Conjugate/ADC Related
Product type
– ADC Related
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.
Related Products
1000 in stock