Download Files:
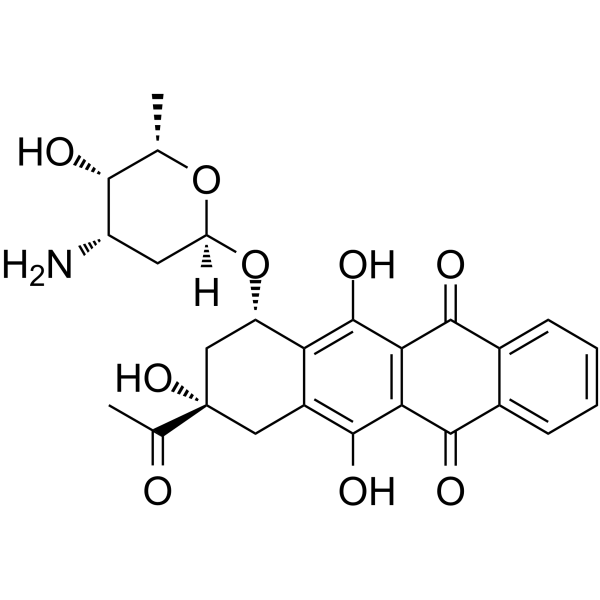
Idarubicin
SKU
HY-17381A-Get quote
Category Reference compound
Tags Anti-infection;Apoptosis;Autophagy;Cell Cycle/DNA Damage, Antibiotic;Autophagy;Bacterial;c-Myc;DNA/RNA Synthesis;Fungal;Topoisomerase, Cancer; Infection
Products Details
Product Description
– Idarubicin is an orally active and potent anthracycline antileukemic agent. Idarubicin inhibits the topoisomerase II interfering with the replication of DNA and RNA transcription. Idarubicin shows induction of DNA damage. Idarubicin inhibits DNA synthesis and of c-myc expression. Idarubicin inhibits the growth of bacteria and yeasts[1][2][3][4][5].
Web ID
– HY-17381A
Shipping
– Room temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C26H27NO9
Citations
– Mol Carcinog. 2023 Jul 26.|Anal Chem. 2022 Oct 4;94(39):13623-13630.|bioRxiv. 2023 Jan 13.|Cancer Lett. 2019 Oct 1;461:31-43. |Exp Cell Res. 2020 Aug 1;393(1):112054.|J Mol Med (Berl). 2019 Aug;97(8):1183-1193.|J Virol. 2019 May 15;93(11):e02230-18. |Nucleic Acids Res. 2018 Apr 20;46(7):3284-3297.|Viruses. 2020 Jun 10;12(6):628.
References
– [1]Orlandi P, et al. Idarubicin and idarubicinol effects on breast cancer multicellular spheroids. J Chemother. 2005 Dec;17(6):663-7.|[2]Robert J. Clinical pharmacokinetics of idarubicin. Clin Pharmacokinet. 1993 Apr;24(4):275-88.|[3]Siegsmund MJ, et al. Enhanced in vitro cytotoxicity of idarubicin compared to epirubicin and doxorubicin in rat prostate carcinoma cells. Eur Urol. 1997;31(3):365-70.|[4]Gewirtz DA, et al. Induction of DNA damage, inhibition of DNA synthesis and suppression of c-myc expression by the anthracycline analog, idarubicin (4-demethoxy-daunorubicin) in the MCF-7 breast tumor cell line. Cancer Chemother Pharmacol. 1998;41(5):361-|[5]Kinnunen U, et al. Idarubicin inhibits the growth of bacteria and yeasts in an automated blood culture system. Eur J Clin Microbiol Infect Dis. 2009 Mar;28(3):301-3.
CAS Number
– 58957-92-9
Molecular Weight
– 497.49
SMILES
– OC(C(C(C1=CC=CC=C21)=O)=C3C2=O)=C4[C@H](C[C@@](C(C)=O)(O)CC4=C3O)O[C@@](O[C@@H](C)[C@H]5O)([H])C[C@@H]5N
Clinical Information
– Launched
Research Area
– Cancer; Infection
Solubility
– 10 mM in DMSO
Target
– Antibiotic;Autophagy;Bacterial;c-Myc;DNA/RNA Synthesis;Fungal;Topoisomerase
Isoform
– Topo II
Pathway
– Anti-infection;Apoptosis;Autophagy;Cell Cycle/DNA Damage
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.