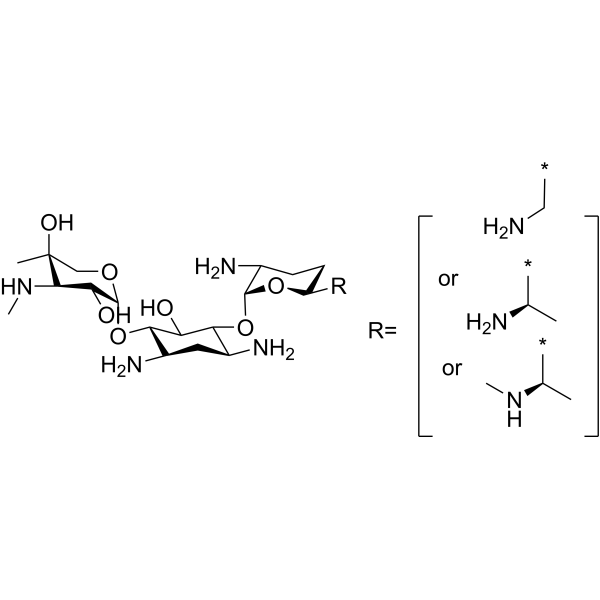
Download Files:
Gentamicin
SKU
HY-A0276A-Get quote
Category Reference compound
Tags Anti-infection, Antibiotic;Bacterial, Infection
Products Details
Product Description
– Gentamicin, an orally active aminoglycoside antibiotic, inhibits the growth of both gram-positive and gram-negative bacteria and to inhibit several strains of mycoplasma in tissue culture. Gentamicin inhibits DNase I with an IC50 of 0.57 mM[1][2][3][4].
Web ID
– HY-A0276A
Shipping
– Room temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C(19-21)H(39-43)N5O7
Citations
– Biomed Pharmacother. 2023 Nov 8:115856.|Curr Microbiol. 2023 May 31;80(7):230.|Food Chem. 2022 Sep 26;403:134399.|Int J Med Microbiol. 2023 Mar 28;313(2):151578.|Nat Microbiol. 2023 Mar;8(3):410-423.|ACS Appl Mater Interfaces. 2019 Oct 16;11(41):38190-38204. |Am J Physiol Cell Physiol. 2019 Aug 1;317(2):C277-C286.|Appl Microbiol Biotechnol. 2022 Apr;106(7):2689-2702.|Aquaculture. 2021, 736248.|Cell Death Dis. 2023 Jan 11;14(1):15.|Cell Transplant. Jan-Dec 2021;30:963689721997151.|Evid Based Complement Alternat Med. 2015;2015:639412.|J Ethnopharmacol. 2020 Sep 15;259:112882. |Mol Immunol. 2017 Dec;92:151-160.|Mol Med Rep. 2016 Dec;14(6):5304-5310.|Mutat Res Genet Toxicol Environ Mutagen. 2023 Apr 5,503636.|Nat Commun. 2022 Mar 2;13(1):1116.|Research Square Print. October 6th, 2022.|Toxicology. 2022 Nov 29;483:153386.
References
– [1]Xu W, et al. A rapid and sensitive method for kinetic study and activity assay of DNase I in vitro based on a GO-quenched hairpin probe. Anal Bioanal Chem. 2016 May;408(14):3801-9.|[2]Rudin A, et al. Antibacterial activity of gentamicin sulfate in tissue culture. Appl Microbiol. 1970 Dec;20(6):989-90.|[3]Kumar CG, et al. Microbial biosynthesis and applications of gentamicin: a critical appraisal. Crit Rev Biotechnol. 2008;28(3):173-212.|[4]Espersen F, et al. Effect of treatment with methicillin and gentamicin in a new experimental mouse model of foreignbody infection. Antimicrob Agents Chemother. 1994 Sep;38(9):2047-53.
CAS Number
– 1403-66-3
Molecular Weight
– N/A
SMILES
– O[C@]1(C)C(NC)[C@@H](O)[C@@H](O[C@H]2C(N)C[C@H](N)[C@@H](O[C@@H]3[C@H](N)CC[C@@H]([R])O3)[C@@H]2O)OC1.NC[*].C[C@H]([*])N.C[C@H]([*])NC.[R=].[or].[or]
Clinical Information
– Launched
Research Area
– Infection
Solubility
– 10 mM in DMSO
Target
– Antibiotic;Bacterial
Isoform
– Aminoglycoside
Pathway
– Anti-infection
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





