Download Files:
Darusentan
SKU
HY-15404-1 mg
Category Reference compound
Tags Cardiovascular Disease; Endocrinology, Endothelin Receptor, GPCR/G Protein
$61 – $1,100
Products Details
Product Description
– Darusentan (Lu-135252) is a selective endothelin receptor A (ET-A) receptor antagonist, which binds with a Ki of 1.4 nM to the ET-A receptor and a Ki of 184 nM to ET-B receptor, respectively with a 100-fold selectivity for ETA rather than ETB receptors[1]. Darusentan competes for radiolabeled endothelin binding in rat aortic vascular smooth muscle cells (RAVSMs) membranes with single-site kinetics, exhibiting a Ki of 13 nM[2].
Web ID
– HY-15404
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
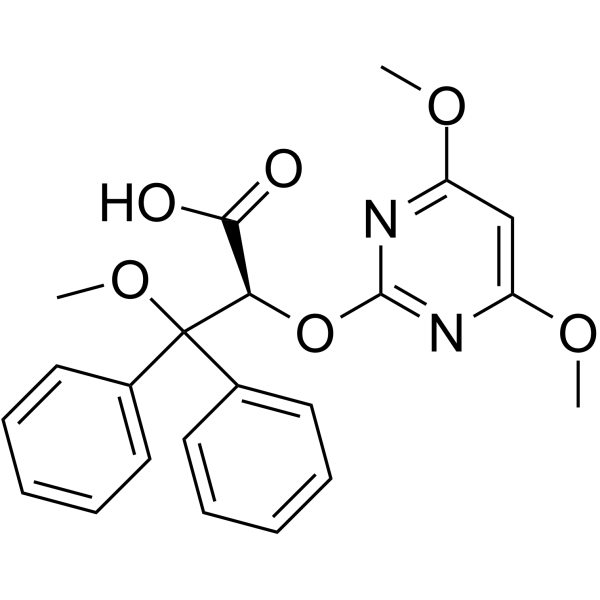
– C22H22N2O6
References
– [1]Frank Enseleit, et al. Darusentan, a selective endothelin A receptor antagonist, for the oral treatment of resistant hypertension. Ther Adv Cardiovasc Dis. 2010 Aug;4(4):231-40.|[2]H H Dao, et al. Norepinephrine-induced aortic hyperplasia and extracellular matrix deposition are endothelin-dependent. J Hypertens. 2001 Nov;19(11):1965-73.|[3]Liang F, et al. Darusentan is a potent inhibitor of endothelin signaling and function in both large and small arteries. Can J Physiol Pharmacol. 2010 Aug;88(8):840-9.
CAS Number
– 171714-84-4
Molecular Weight
– 410.42
Compound Purity
– 98.67
SMILES
– COC1=NC(O[C@H](C(O)=O)C(C2=CC=CC=C2)(C3=CC=CC=C3)OC)=NC(OC)=C1
Clinical Information
– Phase 3
Research Area
– Cardiovascular Disease; Endocrinology
Solubility
– DMSO : 100 mg/mL (ultrasonic)
Target
– Endothelin Receptor
Isoform
– ETA
Pathway
– GPCR/G Protein
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.








