Download Files:
Abemaciclib
$60 – $350
Products Details
Product Description
– Abemaciclib (LY2835219) is a selective CDK4/6 inhibitor with IC50 values of 2 nM and 10 nM for CDK4 and CDK6, respectively.
Web ID
– HY-16297A
Storage Temperature
– 4°C (Powder, protect from light)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C27H32F2N8
Citations
– ACS Appl Mater Interfaces. 2022 May 11;14(18):20628-20640.|Adv Funct Mater. 2021 Apr 30.|Adv Sci (Weinh). 2020 Aug 4;7(18):2000906.|Adv Sci (Weinh). 2022 Aug 2;e2201834.|Bioact Mater. 8 September 2021.|Biochem Biophys Res Commun. 20 December 2021.|Biomed Chromatogr. 2020 Jun;34(6):e4825.|bioRxiv. 2023 Jan 25.|bioRxiv. 2023 Jul 17.|bioRxiv. 2023 Jul 19.|Blood Cancer J. 2022 Jan 11;12(1):5.|Br J Cancer. 2022 Jan 14.|Breast Cancer Res. 2019 Dec 26;21(1):150.|Cancer Discov. 2023 Dec 4.|Cancer Res. 2017 May 1;77(9):2488-2499.|Cancer Res. 2019 Oct 15;79(20):5245-5259.|Cancer Res. 2022 May 16;82(10):1890-1908.|Cell Chem Biol. 2019 Aug 15;26(8):1067-1080.e8. |Cell Death Dis. 2020 Oct 28;11(10):925.|Cell Rep. 2022 Dec 20;41(12):111826.|Cell. 2023 Jun 8;186(12):2628-2643.e21.|Commun Biol. 2021 Mar 25;4(1):399.|Department of Biochemistry. 2020 Oct.|Eur J Drug Metab Pharmacokinet. 2021 Jul 18;1-11.|Front Oncol. 2021 Jul 13;11:704042.|Fundam Clin Pharmacol. 2021 Feb 1.|Heliyon. 2023 Sep 13.|Int J Biol Sci. 2019 Jan 1;15(3):522-532. |Int J Mol Sci. 2021 Jan 8;22(2):E575.|Int J Mol Sci. 2022 Feb 24;23(5):2493.|J Cell Biochem. 2023 Aug 11.|J Med Chem. 2023 Mar 6.|J Oncol. 2022 Jun 23;2022:8724933.|JCI Insight. 2021 Dec 21;e154402.|Leuk Res. September 2022, 106920.|Nat Commun. 2021 Aug 25;12(1):5112.|Nat Commun. 2021 Nov 16;12(1):6607.|Nat Commun. 2022 Aug 10;13(1):4689.|Nat Metab. 2020 Jan;2(1):41-49.|Nature Cancer. 2021 Apr;2(4):429-443.|Naunyn Schmiedebergs Arch Pharmacol. 2023 Feb 4.|NPJ Precis Oncol. 2021 Mar 19;5(1):20.|Patent. US20200108066A1|R Soc Open Sci. 2019 Jan 23;6(1):181714.|Sci Rep. 2022 Jul 20;12(1):12420.|Sci Rep. 2019 Oct 22;9(1):15099. |SSRN. 2023 Sep 29.|Biochem Biophys Rep. 27, September 2021, 101099|Biochem Biophys Res Commun. 2018 Sep 26;504(1):231-237. |Biochem Pharmacol. 2017 Jan 15;124:29-42.|Biochim Biophys Acta. 2017 Nov 20;1865(2):354-363.|bioRxiv. 2020 Jun.|Cancer Res. 2016 Nov 15;76(22):6723-6734. |Cancer Res. 2023 Jun 29;CAN-23-0705.|Cell Death Dis. 2019 Mar 20;10(4):271.|Cell Rep. 2017 Oct 31;21(5):1386-1398.|Cell Rep. 2022 Sep 13;40(11):111331.|Cell. 2018 Nov 1;175(4):984-997.e24.|EMBO J. 2022 Jan 5;e108946.|Harvard Medical School LINCS LIBRARY|J Exp Clin Cancer Res. 2022 Apr 21;41(1):149.|J Oncol. 2019 Jun 2;2019:5952836. |Mol Cell. 2017 Oct 19;68(2):336-349.e6.|Mol Oncol. 2017 Aug;11(8):1035-1049.|Nat Commun. 2017 Jun 27;8:15916.|Nat Commun. 2019 Jun 28;10(1):2860.|Nature. 2017 Aug 24;548(7668):471-475. |Nutrition. 2019 Apr;60:217-226. |Oncotarget. 2017 Jul 27;8(56):95116-95134. |Oncotarget. 2017 Jun 27;8(40):67422-67438. |Sci Transl Med. 2018 Jul 18;10(450):eaaq1093.|STAR Protocols. 2020 Jun 3;1(1):100024.
References
– [1]Ku BM, et al. The CDK4/6 inhibitor LY2835219 has potent activity in combination with mTOR inhibitor in head and neck squamous cell carcinoma. Oncotarget.?2016 Mar 22;7(12):14803-13.|[2]Gelbert LM, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with NSC 613327. Invest New Drugs. 2014 Oct;32(5):825-37.|[3]Yadav V, et al. The CDK4/6 inhibitor LY2835219 overcomes PLX4032 resistance resulting from MAPK reactivation and cyclin D1 upregulation. Mol Cancer Ther. 2014 Oct;13(10):2253-63.
CAS Number
– 1231929-97-7
Molecular Weight
– 506.59
Compound Purity
– 99.96
SMILES
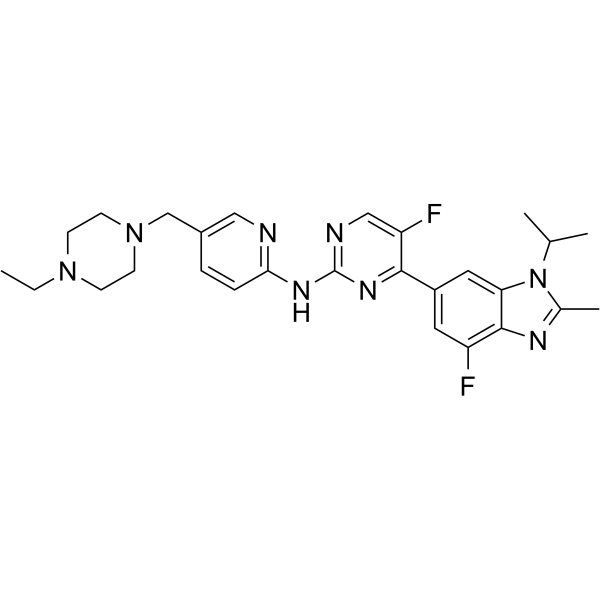
– CC1=NC2=C(F)C=C(C3=NC(NC4=NC=C(CN5CCN(CC)CC5)C=C4)=NC=C3F)C=C2N1C(C)C
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– DMSO : 2.94 mg/mL (ultrasonic;warming;heat to 80°C)|H2O : < 0.1 mg/mL (ultrasonic;warming;heat to 60°C)
Target
– CDK
Isoform
– CDK1;CDK2;CDK4;CDK5;CDK6;CDK7;CDK9
Pathway
– Cell Cycle/DNA Damage
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.







