Download Files:
Zafirlukast
SKU
HY-17492-100 mg
Category Reference compound
Tags GPCR/G Protein, Inflammation/Immunology; Cancer, Leukotriene Receptor
$114 – $445
Products Details
Product Description
– Zafirlukast (ICI 204219) is a potent orally active leukotriene D4 (LTD4) receptor antagonist. Zafirlukast shows anti-asthmatic, anti-inflammatory and anti-bacterial effects.
Web ID
– HY-17492
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
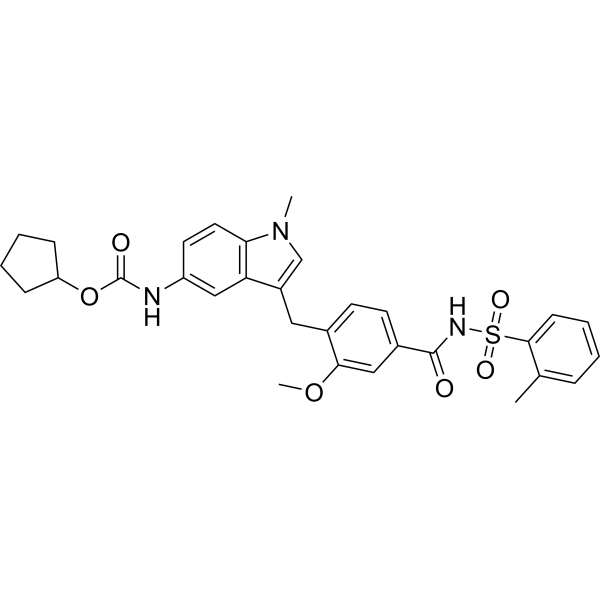
– C31H33N3O6S
References
– [1]Finnerty JP, et al. Role of leukotrienes in exercise-induced asthma. Inhibitory effect of ICI 204219, a potent leukotriene D<sub>4</sub> receptor antagonist. Am Rev Respir Dis. 1992 Apr;145(4 Pt 1):746-9.|[2]Gunning WT, et al. Chemoprevention by lipoxygenase and leukotriene pathway inhibitors of vinyl carbamate-induced lung tumors in mice. Cancer Res. 2002 Aug 1;62(15):4199-201.|[3]Lei C, et al. Zafirlukast attenuates advanced glycation end-products (AGEs)-induced degradation of articular extracellular matrix (ECM). Int Immunopharmacol. 2019;68:68-73.
CAS Number
– 107753-78-6
Molecular Weight
– 575.68
Compound Purity
– 99.89
SMILES
– O=C(OC1CCCC1)NC2=CC3=C(N(C)C=C3CC4=CC=C(C(NS(=O)(C5=CC=CC=C5C)=O)=O)C=C4OC)C=C2
Clinical Information
– Launched
Research Area
– Inflammation/Immunology; Cancer
Solubility
– DMSO : 100 mg/mL (ultrasonic)
Target
– Leukotriene Receptor
Isoform
– LTD4/CysLT1
Pathway
– GPCR/G Protein
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






