Download Files:
Vaniprevir
SKU
HY-10243-Get quote
Category Reference compound
Tags Anti-infection;Metabolic Enzyme/Protease, HCV;HCV Protease, Infection
Products Details
Product Description
– Vaniprevir (MK-7009) is a non-covalent competitive inhibitor of the hepatitis C virus (HCV) NS3/4A protease.
Web ID
– HY-10243
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C38H55N5O9S
References
– [1]Liverton, Nigel J.; Carroll, Steven S.; Di Muzio, Jillian;.MK-7009, a potent and selective inhibitor of hepatitis C virus NS3/4A protease. Antimicrobial Agents and Chemotherapy (2009), Volume Date 2010, 54(1), 305-311.|[2]Kong J, Chen CY, Balsells-Padros J, Cao Y, Dunn RF, Dolman SJ, Janey J, Li H, Zacuto MJ.Synthesis of the HCV protease inhibitor Vaniprevir (MK-7009) using ring-closing metathesis strategy.J Org Chem. 2012 Apr 20;77(8):3820-8. Epub 2012 Apr 10.|[3]Manns MP, Gane E, Rodriguez-Torres M, Stoehr A, Yeh CT, Marcellin P, Wiedmann RT, Hwang PM, Caro L, Barnard RJ, Lee AW; for the MK-7009 Protocol 007 Study Group.Vaniprevir with pegylated interferon alpha-2a and ribavirin in treatment-na?ve patients with chronic hepatitis C: A randomized phase II study.Hepatology. 2012 Sep;56(3):884-893.|[4]Song ZJ, Tellers DM, Journet M, Kuethe JT, Lieberman D, Humphrey G, Zhang F, Peng Z, Waters MS, Zewge D, Nolting A, Zhao D, Reamer RA, Dormer PG, Belyk KM, Davies IW, Devine PN, Tschaen DM.Synthesis of vaniprevir (MK-7009): lactamization to prepare a 20-membered [corrected] macrocycle.J Org Chem. 2011 Oct 7;76(19):7804-15. Epub 2011 Aug 31.|[5]McCauley JA, McIntyre CJ, Rudd MT, Nguyen KT, Romano JJ, Butcher JW, Gilbert KF, Bush KJ, Holloway MK, Swestock J, Wan BL, Carroll SS, DiMuzio JM, Graham DJ, Ludmerer SW, Mao SS, Stahlhut MW, Fandozzi CM, Trainor N, Olsen DB, Vacca JP, Liverton NJ.Discovery of vaniprevir (MK-7009), a macrocyclic hepatitis C virus NS3/4a protease inhibitor.J Med Chem. 2010 Mar 25;53(6):2443-63.
CAS Number
– 923590-37-8
Molecular Weight
– 757.9364
Compound Purity
– 99.60
SMILES
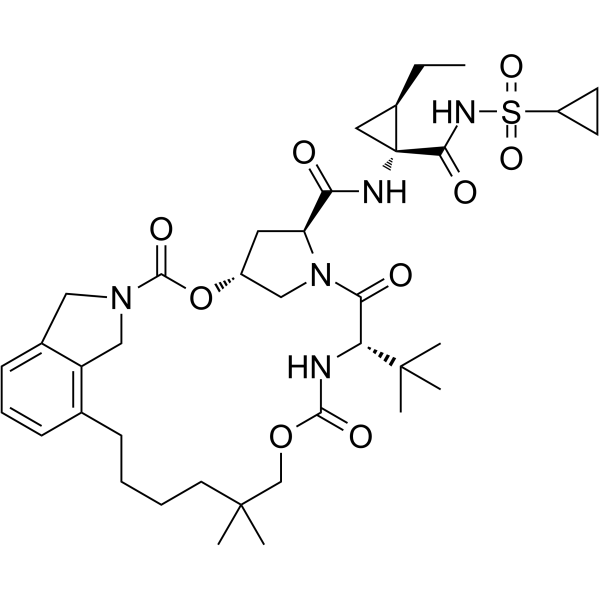
– O=S(NC([C@@]1(NC([C@H]2N(C([C@@H](NC(OCC(CCCC3)(C)C)=O)C(C)(C)C)=O)C[C@H](OC(N4CC5=C3C=CC=C5C4)=O)C2)=O)C[C@H]1CC)=O)(C6CC6)=O
Clinical Information
– Launched
Research Area
– Infection
Solubility
– 10 mM in DMSO
Target
– HCV;HCV Protease
Pathway
– Anti-infection;Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.







