Download Files:
Upamostat
SKU
HY-16511-10 mg
Category Reference compound
Tags Cancer, Metabolic Enzyme/Protease, PAI-1;Ser/Thr Protease
$300 – $1,350
Products Details
Product Description
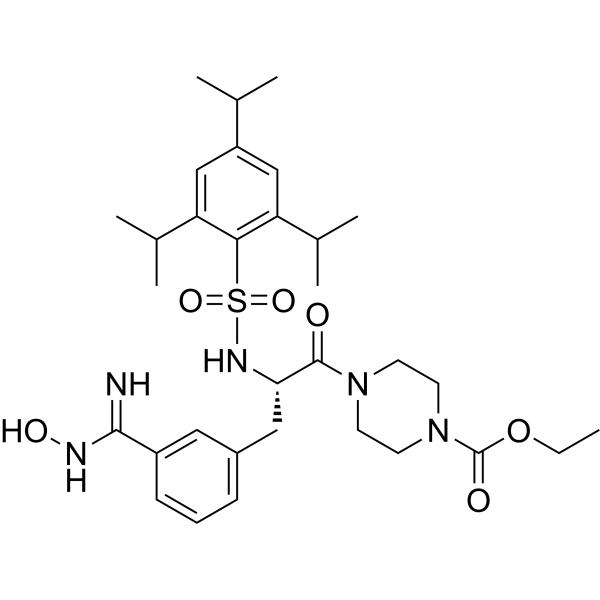
– Upamostat (WX-671) is a serine protease inhibitor. Upamostat is the orally available prodrug of the WX-UK1, which is a urokinase plasminogen activator (uPA) inhibitor.
Web ID
– HY-16511
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C32H47N5O6S
References
– [1]Heinemann V, et al. Phase II randomised proof-of-concept study of the urokinase inhibitor upamostat (WX-671) in combination with gemcitabine compared with gemcitabine alone in patients with non-resectable, locally advanced pancreatic cancer. Br J Cancer. 2013 Mar 5;108(4):766-70.|[2]Froriep D, et al. Activation of the anti-cancer agent upamostat by the mARC enzyme system. Xenobiotica. 2013 Sep;43(9):780-4.|[3]Park C, et al. HPLC-MS/MS analysis of mesupron and its application to a pharmacokinetic study in rats. J Pharm Biomed Anal. 2018 Feb 20;150:39-42.
CAS Number
– 590368-25-5
Molecular Weight
– 629.81
Compound Purity
– 98.0
SMILES
– O=C(N1CCN(C([C@@H](NS(=O)(C2=C(C(C)C)C=C(C(C)C)C=C2C(C)C)=O)CC3=CC=CC(C(NO)=N)=C3)=O)CC1)OCC
Clinical Information
– Phase 3
Research Area
– Cancer
Solubility
– DMSO : ≥ 250 mg/mL
Target
– PAI-1;Ser/Thr Protease
Pathway
– Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






