Download Files:
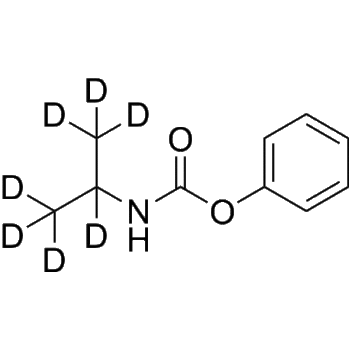
Ulipristal acetate-d6
Products Details
Product Description
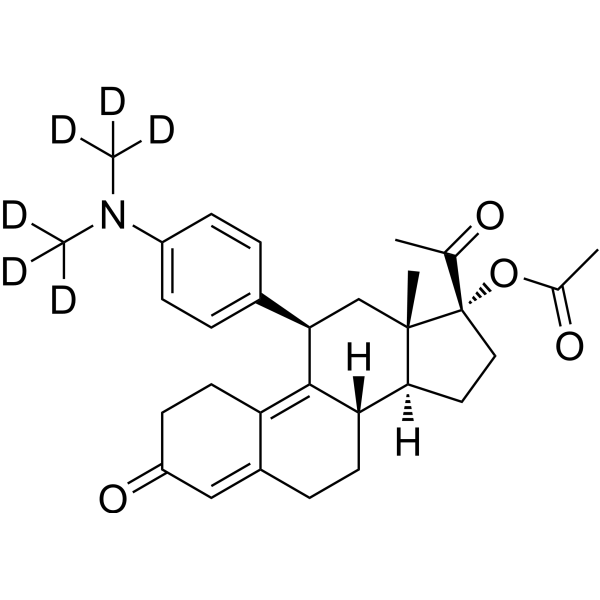
– Ulipristal acetate-d6 is deuterium labeled Ulipristal acetate. Ulipristal acetate (CDB-2914) is an orally active, selective progesterone receptor modulator (SPRM). Ulipristal acetate stimulates the autophagic response selectively in leiomyoma cells. Ulipristal acetate has the potential for benign gynecological conditions treatment, such as uterine myoma[1][2].
Web ID
– HY-16508S
Shipping
– Room temperature
Applications
– Metabolism-protein/nucleotide metabolism
Molecular Formula
– C30H31D6NO4
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216.|[2] Attardi BJ, et al. In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol.|[3]Ciarmela P, et al. Ulipristal acetate modulates the expression and functions of activin a in leiomyoma cells. Reprod Sci. 2014 Sep;21(9):1120-5.|[4]Del Bello B, et al. Autophagy up-regulation by ulipristal acetate as a novel target mechanism in the treatment of uterine leiomyoma: an in vitro study. Fertil Steril. 2019 Dec;112(6):1150-1159.|[5]Hild SA, et al. CDB-2914: anti-progestational/anti-glucocorticoid profile and post-coital anti-fertility activity in rats and rabbits. Hum Reprod. 2000 Apr;15(4):822-9.|[6]Jadav SP, et al. Ulipristal acetate, a progesterone receptor modulator for emergency contraception. J Pharmacol Pharmacother. 2012 Apr;3(2):109-11.|[7]Pohl O, et al. A 39-week oral toxicity study of ulipristal acetate in cynomolgus monkeys. Regul Toxicol Pharmacol. 2013 Jun;66(1):6-12.|[8]Pohl O, et al. Carcinogenicity and chronic rodent toxicity of the selective progesterone receptor modulator ulipristal acetate. Curr Drug Saf. 2013 Apr;8(2):77-97.
CAS Number
– 1621894-64-1
Molecular Weight
– 481.66
SMILES
– O=C1CCC2=C3[C@@]([H])(CCC2=C1)[C@@]([H])([C@@]4(C[C@@H]3C5=CC=C(C=C5)N(C([2H])([2H])[2H])C([2H])([2H])[2H])C)CC[C@@]4(C(C)=O)OC(C)=O
Clinical Information
– No Development Reported
Research Area
– Endocrinology; Cancer
Solubility
– 10 mM in DMSO
Target
– Autophagy;Isotope-Labeled Compounds;Progesterone Receptor
Pathway
– Autophagy;Others;Vitamin D Related/Nuclear Receptor
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.