Download Files:
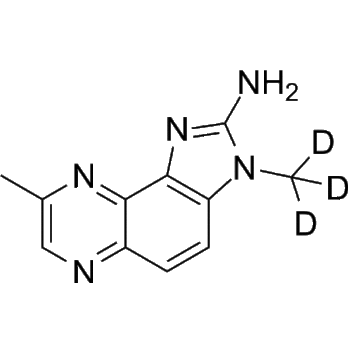
Tropicamide-d3
SKU
HY-B0321S-1 mg
Category Isotope-Labeled Compounds
Tags GPCR/G Protein;Neuronal Signaling, mAChR, Neurological Disease
$400 – $1,600
Products Details
Product Description
– Tropicamide-d3 is the deuterium labeled Tropicamide[1]. Tropicamide (Ro 1-7683) is a selective M4 muscarinic acetylcholine receptor antagonist. Tropicamide produces short acting mydriasis (dilation of the pupil) and cycloplegia when applied as eye drops[2][3].
Web ID
– HY-B0321S
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Neuroscience-Neuromodulation
Molecular Formula
– C17H17D3N2O2
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019 Feb;53(2):211-216.|[2]Lazareno, S., N.J. Buckley, and F.F. Roberts, Characterization of muscarinic M4 binding sites in rabbit lung, chicken heart, and NG108-15 cells. Mol Pharmacol, 1990. 38(6): p. 805-15.|[3]Hernandez, M., et al., Different muscarinic receptor subtypes mediating the phasic activity and basal tone of pig isolated intravesical ureter. Br J Pharmacol, 1993. 110(4): p. 1413-20.
CAS Number
– 2673270-13-6
Molecular Weight
– 287.37
Compound Purity
– 99.10
SMILES
– [2H]C([2H])([2H])CN(CC1=CC=NC=C1)C(C(C2=CC=CC=C2)CO)=O
Clinical Information
– Launched
Research Area
– Neurological Disease
Solubility
– 10 mM in DMSO
Target
– mAChR
Pathway
– GPCR/G Protein;Neuronal Signaling
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.