Download Files:
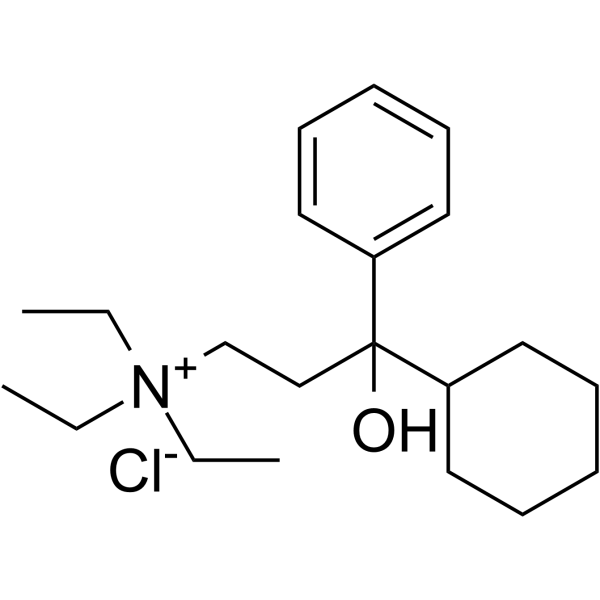
Tridihexethyl (chloride)
SKU
HY-B1806A-1 mg
Category Reference compound
Tags GPCR/G Protein;Neuronal Signaling, Inflammation/Immunology; Neurological Disease, mAChR
$170
Only 1000 item(s) left in stock.
Products Details
Product Description
– Tridihexethyl (Pathilon) chloride is an orally active anticholinergic agent and mAChR antagonist, shows activities of antimuscarinic and anticholinergic. Tridihexethyl chloride shows pronounced antispasmodic and antisecretory effects on the gastrointestinal tract. Tridihexethyl chloride can be used in studies of peptic ulcer disease and acquired nystagmus [1][2].
Web ID
– HY-B1806A
Storage Temperature
– 4°C (Powder, protect from light, stored under nitrogen)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C21H36ClNO
References
– [1]Jabbari B, et al. ffectiveness of trihexyphenidyl against pendular nystagmus and palatal myoclonus: evidence of cholinergic dysfunction. Mov Disord. 1987;2(2):93-8.|[2]Leigh RJ, et al. Effect of anticholinergic agents upon acquired nystagmus: a double-blind study of trihexyphenidyl and tridihexethyl chloride. Neurology. 1991 Nov;41(11):1737-41.
CAS Number
– 4310-35-4
Molecular Weight
– 353.97
Compound Purity
– 96.0
SMILES
– OC(CC[N+](CC)(CC)CC)(C1CCCCC1)C2=CC=CC=C2.[Cl-]
Clinical Information
– Launched
Research Area
– Inflammation/Immunology; Neurological Disease
Solubility
– 10 mM in DMSO
Target
– mAChR
Pathway
– GPCR/G Protein;Neuronal Signaling
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






