Download Files:
Trametinib (DMSO solvate)
SKU
HY-10999A-100 mg
Category Reference compound
Tags Apoptosis;MAPK/ERK Pathway, Apoptosis;MEK, Cancer
$32 – $100
Products Details
Product Description
– Trametinib (DMSO solvate) (GSK-1120212 (DMSO solvate);JTP-74057 (DMSO solvate)) is an orally active MEK inhibitor that inhibits MEK1 and MEK2 with IC50s of about 2 nM. Trametinib (DMSO solvate) activates autophagy and induces apoptosis[1][2].
Web ID
– HY-10999A
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C28H29FIN5O5S
Citations
– Cancer Cell. 2020 Mar 16;37(3):387-402.e7. |Cell Rep. 2021 Jul 20;36(3):109410.|Faculty of Biology, University of Barcelona. 2020 Jan.|Mol Cell. 2019 Jan 3;73(1):7-21.e7. |Patent. US20200046706A1.|Patent. US20210401844A1.|Patent. US20220162299A1.|Patent. US20220177583A1.|PLoS One. 2020 Sep 3;15(9):e0235824.|Sci Signal. 2018 Oct 30;11(554):eaar6795.|ACS Comb Sci. 2019 Dec 9;21(12):805-816.|Acta Pharmacol Sin. 2023 Feb 1.|Adv Sci (Weinh). 2023 Mar 8;e2201164.|Am J Digest Dis. 2015;2(2):95-99.|Am J Respir Cell Mol Biol. 2019 Sep;61(3):355-366.|Aquaculture. 2023 Sep 23, 740148.|Biochem Biophys Res Commun. 2017 Aug 5;489(4):484-489.|Biochem Biophys Res Commun. 2018 Jan 8;495(2):1846-1850. |Biomaterials. 16 September 2022.|Biomaterials. 2018 Sep;178:158-169.|Biomolecules. 2021 Mar 30;11(4):518.|Biomolecules. 2023 Apr 25, 13(5), 740.|bioRxiv. 2019 Sep.|bioRxiv. 2023 Oct 23.|bioRxiv. 2023 Oct 6.|bioRxiv. 2023 Sep 3.|bioRxiv. 2023 Sep 8.|Br J Cancer. 2023 May 17.|Cancer Biol Ther. 2019;20(5):653-665.|Cancer Cell. 2021 Aug 9;39(8):1135-1149.e8.|Cancer Cell. 2021 May 10;39(5):678-693.e11.|Cancer Discov. 2012 Oct;2(10):934-47.|Cancer Discov. 2015 Sep;5(9):960-71.|Cancer Discov. 2018 Mar;8(3):354-369.|Cancer Discov. 2020 Aug;10(8):1226-1239.|Cancer Discov. 2020 Jun;10(6):872-887.|Cancer Immunol Res. 2020 Dec 10.|Cancer Lett. 2017 Nov 1;408:43-54. |Cancer Res. 2022 May 18;canres.4152.2021.|Cancers (Basel). 2022 Mar 11;14(6):1451.|Cancers (Basel). 2023 Apr 13, 15(8), 2280.|Cancers. 2021 Feb 2;13(3):581.|Cell Chem Biol. 2018 Aug 16;25(8):996-1005.e4.|Cell Death Differ. 2020 May;27(5):1520-1538. |Cell Death Dis. 2022 Oct 3;13(10):844.|Cell Death Discov. 2023 Sep 19;9(1):347.|Cell Metab. 2019 Jan 8;29(1):141-155.e9. |Cell Mol Life Sci. 2023 Mar 18;80(4):100.|Cell Oncol. 2022 Oct 21.|Cell Rep. 2019 Jul 2;28(1):119-131.e4. |Cell Syst. 2019 Jul 24;9(1):35-48.e5. |Cell Syst. 2020 Jan 22;10(1):66-81.e11.|Cell Syst. 2020 Nov 18;11(5):478-494.e9.|Cell. 2018 Aug 9;174(4):843-855.e19.|Cells. 2022, 11(15), 2402.|Clin Cancer Res. 2014 Nov 1;20(21):5483-95. |Clin Cancer Res. 2022 Jul 7;ccr.22.1052.|Clin Cancer Res. 2023 Jan 17;CCR-22-1973.|Comput Struct Biotechnol J. 2019 Feb 8;17:352-361.|Dis Model Mech. 2023 Mar 2;dmm.049769.|EBioMedicine. 2020 Jan;51:102583.|EBioMedicine. 2022 Dec 8;87:104397.|Eur J Cancer. 2018 Aug;99:37-48.|Exp Cell Res. 2020 Aug 1;393(1):112054.|Exp Mol Med. 2023 Oct 2.|Front Cell Dev Biol. 2018 Sep 25;6:111.|Front Oncol. 2021 Jul 13;11:704042.|Genes Dis. 1 July 2021.|Geroscience. 2023 Sep 26.|Harvard Medical School LINCS LIBRARY|Inflammation. 2023 May 12.|Int J Cancer. 2019 Mar 15;144(6):1379-1390.|Int J Mol Sci. 2022 Nov 19;23(22):14385.|Int J Mol Sci. 2022, 23(19), 11939.|Int J Mol Sci. 2022, 23(20), 12587.|Int J Mol Sci. 2023 Mar 13.|J Cardiothorac Surg. 2021 May 10;16(1):127.|J Cell Biochem. 2016 Jun;117(6):1340-51. |J Cell Sci. 2019 May 31;132(11):jcs224071. |J Clin Invest. 2022 Nov 22;e153470.|J Clin Toxicol 2014, 4:5|J Clin Toxicol. 2014 October 4, 4:5.|J Exp Clin Cancer Res. 2018 Sep 5;37(1):218.|J Exp Clin Cancer Res. 2023 Apr 19;42(1):92.|J Hepatol. 2021 Aug;75(2):363-376.|J Invest Dermatol. 2021 Sep 14;S0022-202X(21)01672-9.|J Invest Dermatol. 2023 Mar 30;S0022-202X(23)01952-8.|J Transl Med. 2022 Jun 7;20(1):263.|J Transl Med. 2023 Jan 9;21(1):9.|J Vasc Res. 2023 Feb 2.|Leuk Lymphoma. 2021 Feb;62(2):337-347.|medRxiv. 2023 Nov 13.|Mol Cancer Ther. 2016 Aug;15(8):1859-69. |Mol Cancer. 2023 May 20;22(1):86.|Mol Pharmacol. 2019 Dec;96(6):862-870.|Mol Psychiatry. 2022 Aug 10.|Nat Biomed Eng. 2018 Aug;2(8):578-588.|Nat Cell Biol. 2023 Jan 12.|Nat Commun. 2020 Aug 13;11(1):4053.|Nat Commun. 2020 May 11;11(1):2333.|Nat Commun. 2020 Oct 29;11(1):5463.|Nat Commun. 2021 Aug 25;12(1):5058.|Nat Commun. 2023 May 19;14(1):2859.|Nat Commun. 2019 Jan 17;10(1):296.|Neuro Oncol. 2019 Mar 18;21(4):486-497. |Oncogenesis. 2019 Nov 4;8(11):65. |Oncol Lett. 2017 Dec;14(6):6863-6868.|Oncol Lett. December 17, 2021.|Oncol Rep. 2018 Dec; 40(6): 3313–3322.|Oncotarget. 2015 Oct 13;6(31):31313-22. |Oncotarget. 2017 Feb 28;8(9):14835-14846. |Oncotarget. 2020 Nov 3;11(44):3921-3932.|Patent. US20180161326A1.|Patent. US20180169102A1.|Patent. US20180291421A1.|Patent. US20200276189A1.|Patent. US20200291111A1.|Patent. US20200308623A1.|Patent. US20200354680A1.|Patent. US20210008047A1.|Patent. US20210010089A1.|Patent. US20220265657A1.|Patent. US20220372134A1.|Patent. US20230049752A1.|Patent. US20230068698A1.|Patent. US20230158019A1.|Phytomedicine. 2023 Nov 11, 155208.|PLoS Biol. 2020 Jul 17;18(7):e3000778.|Proc Natl Acad Sci U S A. 2018 Oct 9;115(41):E9570-E9579. |Proc Natl Acad Sci U S A. 2019 Mar 5;116(10):4508-4517.|Research Square Preprint. 2020 Jun.|Research Square Preprint. 2021 Oct.|Research Square Preprint. 2023 Jul 10.|Research Square Preprint. 2023 May 16.|Research Square Print. October 10th, 2022.|Sci Rep. 2016 Oct 19;6:35531.|Sci Rep. 2017 Mar 28;7:45332.|Sci Rep. 2022 Nov 5;12(1):18811.|Sci Transl Med. 2018 Jul 18;10(450):eaaq1093.|Sci Transl Med. 2021 Jan 27;13(578):eaba7308.|SSRN. 2023 Aug 9.|Stem Cell Reports. 2016 Jan 12;6(1):74-84.|Stem Cell Reports. 2017 Dec 12;9(6):1948-1960.|Theranostics. 2022 Oct 3;12(16):7051-7066.|Theranostics. 2020 Feb 18;10(8):3579-3593. |University of California. 2023 Feb.|Viruses. 2021, 13(9), 1758.
References
– [1]Yamaguchi T, et al. Suppressive effect of an orally active MEK1/2 inhibitor in two different animal models for rheumatoid arthritis: a comparison with HWA486. Inflamm Res, 2012, 61(5), 445-454.|[2]Abe H, et al. Discovery of a Highly Potent and Selective MEK Inhibitor: GSK1120212 (JTP-74057 DMSO Solvate). ACS Med Chem Lett. 2011 Feb 28;2(4):320-4.
CAS Number
– 1187431-43-1
Molecular Weight
– 693.53
Compound Purity
– 99.74
SMILES
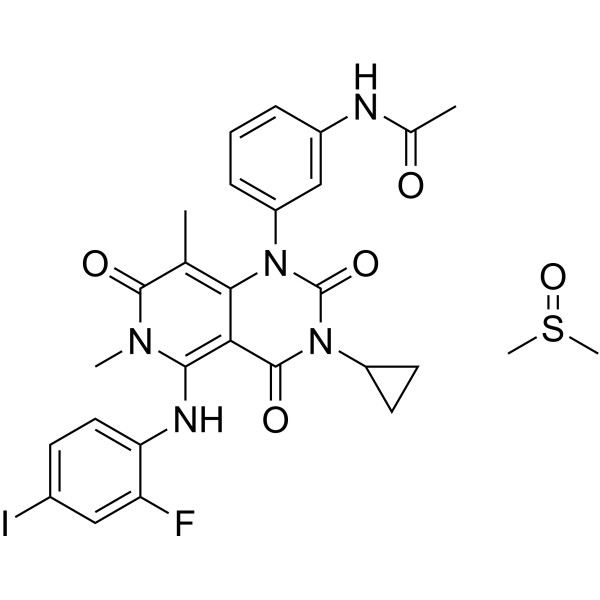
– CN1C(C(C)=C(N(C(N(C2=O)C3CC3)=O)C4=CC=CC(NC(C)=O)=C4)C2=C1NC5=CC=C(C=C5F)I)=O.CS(C)=O
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– DMSO : 3.33 mg/mL (ultrasonic)
Target
– Apoptosis;MEK
Isoform
– MEK1;MEK2
Pathway
– Apoptosis;MAPK/ERK Pathway
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.







