Download Files:
Tolbutamide-d9
SKU
HY-B0401S-1 mg
Category Isotope-Labeled Compounds
Tags Autophagy;Membrane Transporter/Ion Channel, Autophagy;Potassium Channel, Metabolic Disease; Cancer
$260 – $730
Products Details
Product Description
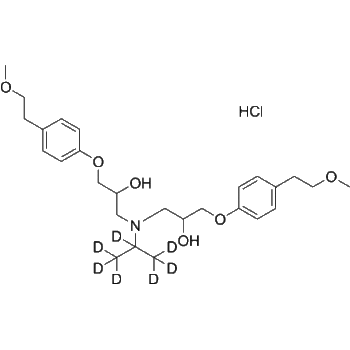
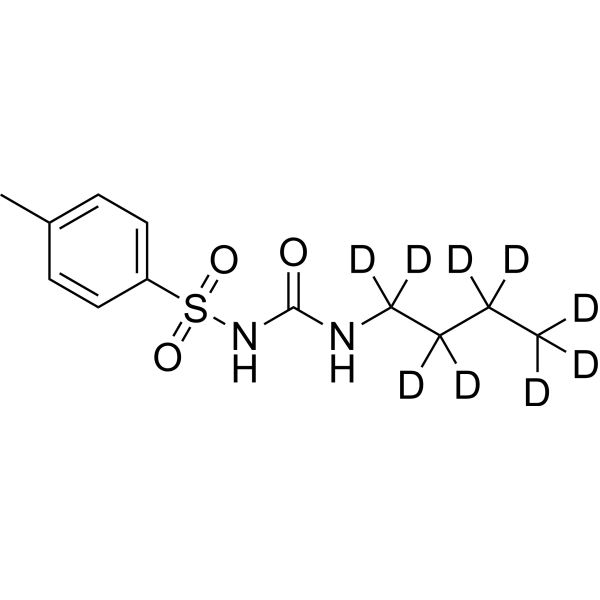
– Tolbutamide-d9 is the deuterium labeled Tolbutamide. Tolbutamide is a first generation potassium channel blocker, sulfonylurea oral hypoglycemic agent.
Web ID
– HY-B0401S
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Molecular Formula
– C12H9D9N2O3S
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216. |[2]Wray, H.L. and A.W. Harris, Adenosine 3′, 5′-monophosphate-dependent protein kinase in adipose tissue: inhibition by tolbutamide. Biochem Biophys Res Commun, 1973. 53(1): p. 291-4.|[3]Sanchez-Alvarez, R., et al., Tolbutamide reduces glioma cell proliferation by increasing connexin43, which promotes the up-regulation of p21 and p27 and subsequent changes in retinoblastoma phosphorylation. Glia, 2006. 54(2): p. 125-34.
CAS Number
– 1219794-57-6
Molecular Weight
– 279.40
Compound Purity
– 99.0
SMILES
– O=S(C1=CC=C(C)C=C1)(NC(NC([2H])([2H])C([2H])([2H])C([2H])([2H])C([2H])([2H])[2H])=O)=O
Clinical Information
– No Development Reported
Research Area
– Metabolic Disease; Cancer
Solubility
– 10 mM in DMSO
Target
– Autophagy;Potassium Channel
Pathway
– Autophagy;Membrane Transporter/Ion Channel
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.