Download Files:
Tofacitinib
SKU
HY-40354-10 mg
Category Reference compound
Tags Apoptosis;Epigenetics;JAK/STAT Signaling;Protein Tyrosine Kinase/RTK;Stem Cell/Wnt, Apoptosis;JAK, Inflammation/Immunology; Cancer
$38 – $336
Products Details
Product Description
– Tofacitinib is an orally available JAK3/2/1 inhibitor with IC50s of 1, 20, and 112 nM, respectively.
Web ID
– HY-40354
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
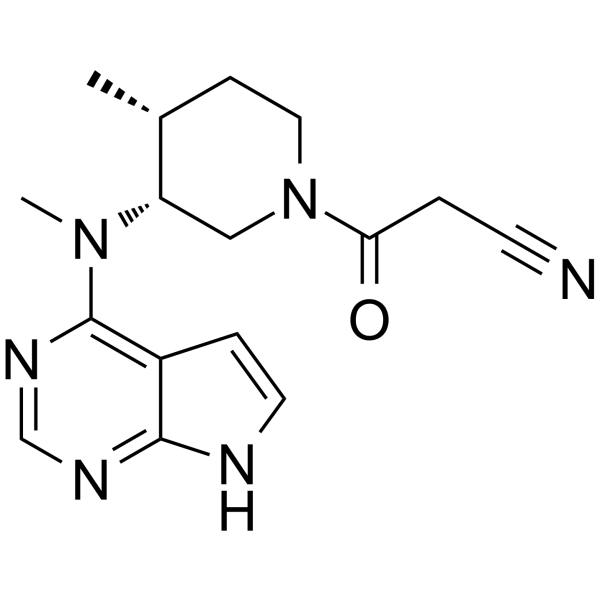
– C16H20N6O
Citations
– Ann Rheum Dis. 2021 Sep;80(9):1201-1208.|Arch Dermatol Res. 2020 Jul;312(5):337-352.|Biochem Pharmacol. 2020 Aug;178:114103.|Biochem Pharmacol. 2023 May 19;213:115618.|Biomed Chromatogr. 2021 Feb 1;e5081.|bioRxiv. 2020 Dec 9;2020.12.09.416586.|BMC Med. 2021 Oct 15;19(1):247.|Cell Rep. 2020 Sep 15;32(11):108158.|Cell Syst. 2018 Apr 25;6(4):424-443.e7.|Chemrxiv. 2023 Aug 3.|Chemrxiv. Aug 11, 2021.|Eur J Drug Metab Pharmacokinet. 2021 Jul 18;1-11.|Exp Cell Res. 2020 Aug 1;393(1):112054.|Exp Cell Res. 2021 Feb 15;399(2):112482.|Food Chem Toxicol. 2020 Dec;146:111835.|Free Radic Biol Med. 2019 Aug 1;139:80-91.|Fundam Clin Pharmacol. 2021 Feb 1.|Inflamm Bowel Dis. 2020 Feb 11;26(3):407-422.|Inflammation. 2023 Jun 13.|iScience. 2021 Sep 25;24(10):103173.|J Biochem Mol Toxicol. 2021 Jul 31;e22851.|J Immunol. 2013 Oct 1;191(7):3568-77. |J Pharmacol Exp Ther. 2019 Aug;370(2):137-147. |Mol Biol Rep. 2022 Dec 12.|Mol Hum Reprod. 2021 Mar 24;27(4):gaab016.|Nano Res. 2023 Apr 15.|Patent. US20180263995A1.|Patent. US20220274992A1.|Phytomedicine. November 2022, 154416.|Phytother Res. 2021 Feb;35(2):1033-1047.|PLoS One. 2018 Jan 10;13(1):e0189247. |Pulm Pharmacol Ther. 2017 Apr;43:60-67. |Research Square Print. 2022 Aug.|Research Square Print. 2023 Mar 14.|Research Square Print. January 9th, 2023.|Respir Res. 2022 Sep 13;23(1):244.|Sci Rep. 2021 Apr 1;11(1):7372.|Sci Rep. 2022 May 12;12(1):7843.|Sci Transl Med. 2018 Jul 18;10(450):eaaq1093.|Cell Mol Gastroenterol Hepatol. 2021 Oct 6;S2352-345X(21)00192-2.|Cell Prolif. 2021 Jan;54(1):e12933.|Int J Rheum Dis. 2020 Aug;23(8):1066-1075.|J Med Chem. 2021 Feb 11.
References
– [1]Jiang JK, et al. Examining the chirality, conformation and selective kinase inhibition of 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile (CP-690,550). J Med Chem. 2008 Dec 25;51(24):8012-8.|[2]Onda M, et al. Tofacitinib suppresses antibody responses to protein therapeutics in murine hosts. J Immunol. 2014 Jul 1;193(1):48-55.|[3]Calama E, et al. Tofacitinib ameliorates inflammation in a rat model of airway neutrophilia induced by inhaled LPS. Pulm Pharmacol Ther. 2017 Apr;43:60-67.|[4]LaBranche TP, et al. JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis Rheum. 2012 Nov;64(11):3531-42.
CAS Number
– 477600-75-2
Molecular Weight
– 312.37
Compound Purity
– 99.99
SMILES
– O=C(CC#N)N1C[C@H](N(C2=C3C(NC=C3)=NC=N2)C)[C@H](C)CC1
Clinical Information
– Launched
Research Area
– Inflammation/Immunology; Cancer
Solubility
– DMSO : 125 mg/mL (ultrasonic)|Ethanol : 2.5 mg/mL (ultrasonic)|H2O : 0.15 mg/mL (ultrasonic;warming)
Target
– Apoptosis;JAK
Isoform
– JAK1;JAK2;JAK3
Pathway
– Apoptosis;Epigenetics;JAK/STAT Signaling;Protein Tyrosine Kinase/RTK;Stem Cell/Wnt
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






