Download Files:
Tandutinib
SKU
HY-10202-100 mg
Category Reference compound
Tags Apoptosis;c-Kit;FLT3;PDGFR, Apoptosis;Protein Tyrosine Kinase/RTK, Cancer
$66 – $106
Products Details
Product Description
– Tandutinib (MLN518) is a potent and selective inhibitor of the FLT3 with an IC50 of 0.22 μM, and also inhibits c-Kit and PDGFR with IC50s of 0.17 μM and 0.20 μM, respectively. Tandutinib can be used for acute myelogenous leukemia (AML)[1][2]. Tandutinib has the ability to cross the blood-brain barrier[3].
Web ID
– HY-10202
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C31H42N6O4
References
– [1]Kelly LM, et al. CT53518, a novel selective FLT3 antagonist for the treatment of acute myelogenous leukemia (AML). Cancer Cell, 2002, 1(5), 421-432.|[2]Griswold IJ, et al. Effects of MLN518, a dual FLT3 and KIT inhibitor, on normal and malignant hematopoiesis. Blood, 2004, 104(9), 2912-2918.|[3]Yang JJ, et al. P-glycoprotein and breast cancer resistance protein affect disposition of tandutinib, a tyrosine kinase inhibitor. Drug Metab Lett. 2010 Dec;4(4):201-12.
CAS Number
– 387867-13-2
Molecular Weight
– 562.70
Compound Purity
– 99.48
SMILES
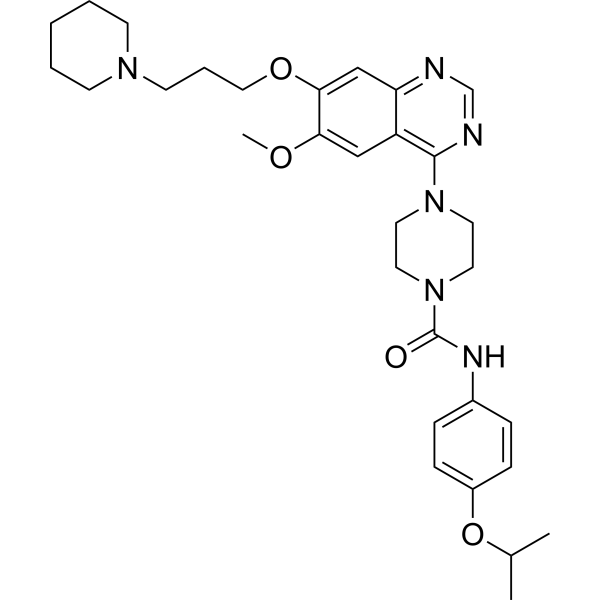
– O=C(N1CCN(C2=C(C(C=C3OCCCN4CCCCC4)=NC=N2)C=C3OC)CC1)NC5=CC=C(OC(C)C)C=C5
Clinical Information
– Phase 2
Research Area
– Cancer
Solubility
– DMSO : 50 mg/mL (ultrasonic)
Target
– Apoptosis;c-Kit;FLT3;PDGFR
Isoform
– PDGFR
Pathway
– Apoptosis;Protein Tyrosine Kinase/RTK
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






