Download Files:
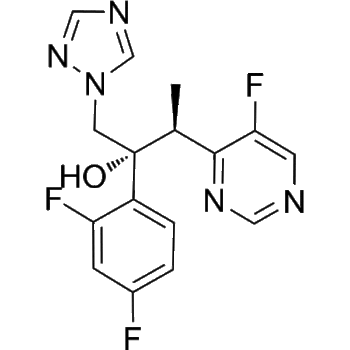
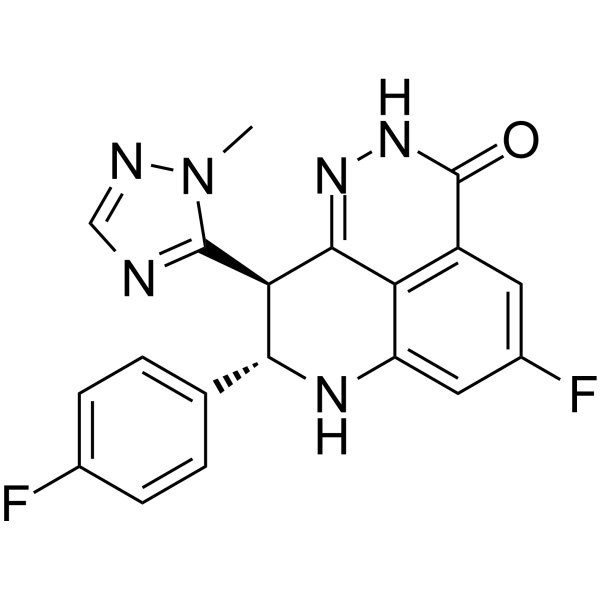
Talazoparib
$70 – $550
Products Details
Product Description
– Talazoparib (BMN-673) is a highly potent, orally active PARP1/2 inhibitor.Talazoparib inhibits PARP1 and PARP2 enzyme activity with Kis of 1.2 nM and 0.87 nM, respectively. Talazoparib has antitumor activity[1].
Web ID
– HY-16106
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C19H14F2N6O
Citations
– ACS Appl Mater Interfaces. 2019 Apr 3;11(13):12342-12356. |Am J Cancer Res. 2020 Aug 1;10(8):2649-2676.|Bioeng Transl Med. 2019 Jun 14;4(2):e10131. |Bioinformatics. 2022 Jul 26;btac533.|Biomed Res Int. 2023 Feb 6.|bioRxiv. 2023 Apr 17.|bioRxiv. 2023 Jun 22.|bioRxiv. 2023 Jun 28.|bioRxiv. April 22, 2021.|BMC Cancer. 2022 Mar 23;22(1):312.|Cancer Cell Int. 2023 Jun 27;23(1):128.|Cancer Cell. 2020 Dec 14;38(6):844-856.e7.|Cancer Discov. 2017 Sep;7(9):984-998.|Cancer Discov. 2022 May 12;candisc.1181.2021.|Cancer Lett. 2021 Jan 22;S0304-3835(21)00024-0.|Cancer Lett. 2021 Mar 1;500:208-219.|Cancer Lett. 2022 Aug 1;215851.|Cancer Res. 2021 Jun 14.|Cancer Res. 2023 Sep 11.|Cell Rep. 2023 Oct 16;42(10):113256.|Clin Cancer Res. 2017 Feb 15;23(4):1001-1011. |Data Brief. 22 September 2021, 107394.|Département de Pharmacologie et Physiologie, Faculté de Médecine. 2020 Jun.|Drug Des Devel Ther. 2020 Feb 25;14:783-793.|EBioMedicine. 2018 Dec;38:47-56. |EBioMedicine. 2020 Sep;59:102923.|Front Oncol. 2021 Jul 9;11:681441.|Genes (Basel). 2023 Jun 20, 14(6), 1295.|Int J Mol Sci. 2022, 23(22), 14338|Int J Mol Sci. 2020 Feb 11;21(4):1185.|Integr Biol (Camb). 2019 Apr 1;11(4):130-141.|Invest New Drugs. 2021 Mar 12.|J Clin Invest. 2021 Jun 1;131(11):146256.|J Med Chem. 2023 Aug 21.|J Pers Med. 2023 Aug 27, 13(9), 1315.|Methods Mol Biol. 2018;1711:351-398.|Mol Cell. 2017 May 18;66(4):503-516.e5. |Nat Cancer. 2022 Oct;3(10):1211-1227.|Nat Commun. 2019 Jun 11;10(1):2556. |Nat Genet. 2022 Dec;54(12):1983-1993.|Neoplasia. 2019 Jul 27;21(9):863-871.|Oncogene. 2017 Aug 17;36(33):4682-4691.|Oncogene. 2021 Sep 10.|Oncol Rep. 2019 Nov;42(5):2097-2107.|Oxid Med Cell Longev. 14 Jul 2022.|Patent. US20180263995A1.|Patent. US20180362972A1.|Patent. US20200078369A1|Patent. US20200129476A1|Patent. US20210030680A1.|Patent. US20220054606A1.|Radiother Oncol. 2021 Jan 29;157:175-181.|Research Square Print. January 4th, 2023.|Sci Adv. 2022 Feb 18;8(7):eabl9794.|Sci Rep. 2023 Feb 27;13(1):3334.|Theranostics. 2020 Jul 25;10(21):9477-9494. |DNA Repair (Amst). 2019 Jan;73:64-70.
References
– [1]Wang B, et al. Discovery and Characterization of (8S,9R)-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-2,7,8,9-tetrahydro-3H-pyrido[4,3,2-de]phthalazin-3-one (BMN 673, Talazoparib), a Novel, Highly Potent, and Orally Efficacious Poly(ADP-ribose) Polymerase-1/2 Inhibitor, as an Anticancer Agent. J Med Chem. 2016 Jan 14;59(1):335-57.|[2]Shen Y, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency.Clin Cancer Res. 2013 Sep 15;19(18):5003-15.
CAS Number
– 1207456-01-6
Molecular Weight
– 380.35
Compound Purity
– 99.89
SMILES
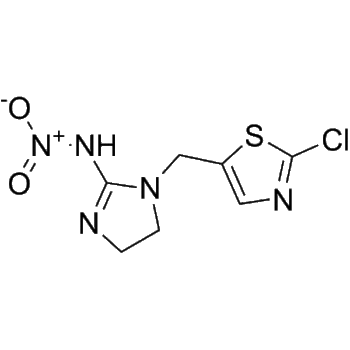
– O=C1NN=C2C3=C1C=C(F)C=C3N[C@H](C4=CC=C(F)C=C4)[C@H]2C5=NC=NN5C
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– DMSO : 25 mg/mL (ultrasonic)
Target
– PARP
Isoform
– PARP1;PARP2
Pathway
– Cell Cycle/DNA Damage;Epigenetics
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.