Download Files:
Suramin (sodium salt)
$300 – $770
Products Details
Product Description
– Suramin sodium salt (Suramin hexasodium salt) is a reversible and competitive protein-tyrosine phosphatases (PTPases) inhibitor[1]. Suramin sodium salt is a potent inhibitor of sirtuins: SirT1 (IC50=297 nM), SirT2 (IC50=1.15 μM), and SirT5 (IC50=22 μM)[2]. Suramin sodium salt is a competitive inhibitor of reverse transcriptase (DNA topoisomerase II: IC50=5 μM)[3][4]. Suramin sodium salt is a potent SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) inhibitor[5]. Suramin sodium salt efficiently inhibits IP5K and is an antiparasitic, anti-neoplastic and anti-angiogenic agent[6][7][8].
Web ID
– HY-B0879A
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture and light)
Shipping
– Room Temperature
Applications
– Cancer-programmed cell death
Molecular Formula
– C51H34N6Na6O23S6
Citations
– Br J Pharmacol. 2021 Aug 6.|J Agric Food Chem. 2023 Sep 19.|J Biol Chem. 2020 Jul 24;295(30):10281-10292. |J Biol Chem. 2021 Sep 3;101166.|J Virol Methods. 2021 Sep 14;298:114283.|Nat Struct Mol Biol. 2021 Mar;28(3):319-325.|Research Square Preprint. 2020 Nov.|Behav Brain Res. 2023 Jun 22;114548.|Biomicrofluidics. 2019 Nov 21;13(6):064117.|Clin Transl Med. 2021 Jun;11(6):e485.|Int Immunopharmacol. 2023 May 12;120:110295.|J Bone Miner Metab. 2023 Jul 7.
References
– [1]Jindal HK, et al. Suramin affects DNA synthesis in HeLa cells by inhibition of DNA polymerases. Cancer Res. 1990 Dec 15;50(24):7754-7.|[2]Izikki M, et al. The beneficial effect of suramin on monocrotaline-induced pulmonary hypertension in rats. PLoS One. 2013 Oct 15;8(10):e77073.|[3]Zhang YL, et al. Suramin is an active site-directed, reversible, and tight-binding inhibitor of protein-tyrosine phosphatases. J Biol Chem. 1998 May 15;273(20):12281-7.|[4]Trapp J, et al. Structure-activity studies on suramin analogues as inhibitors of NAD+-dependent histone deacetylases (sirtuins). ChemMedChem. 2007 Oct;2(10):1419-31.|[5]Schuetz A, et al. Structural basis of inhibition of the human NAD<sup>+</sup>-dependent deacetylase SIRT5 by suramin. Structure. 2007 Mar;15(3):377-89.|[6]De Clercq E, et al. Suramin: a potent inhibitor of the reverse transcriptase of RNA tumor viruses. Cancer Lett. 1979 Nov;8(1):9-22.|[7]Novaes RD, et al. Purinergic Antagonist Suramin Aggravates Myocarditis and Increases Mortality by EnhancingParasitism, Inflammation, and Reactive Tissue Damage in Trypanosoma cruzi-Infected Mice. Oxid Med Cell Longev. 2018 Sep 30;2018:7385639.|[8]Wanchao Yin, et al. Structural basis for inhibition of the SARS-CoV-2 RNA polymerase by suramin. Nat Struct Mol Biol. 2021 Mar;28(3):319-325.|[9]Xiaozhe Zhang, et al. Suramin and NF449 Are IP5K Inhibitors That Disrupt IP6-mediated Regulation of Cullin RING Ligase and Sensitize Cancer Cells to MLN4924/pevonedistat. J Biol Chem. 2020 Jun 3;jbc.RA120.014375.
CAS Number
– 129-46-4
Molecular Weight
– 1429.17
Compound Purity
– 99.48
SMILES
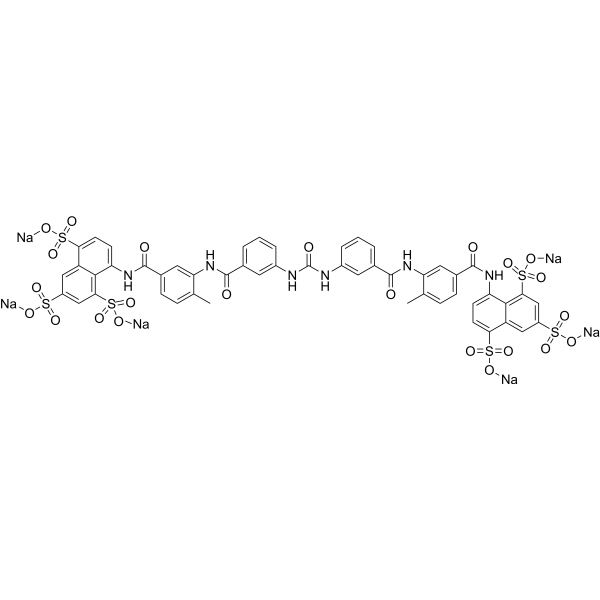
– O=C(NC1=CC(C(NC2=CC(C(NC3=CC=C(S(=O)(O[Na])=O)C4=CC(S(=O)(O[Na])=O)=CC(S(=O)(O[Na])=O)=C34)=O)=CC=C2C)=O)=CC=C1)NC5=CC(C(NC6=CC(C(NC7=CC=C(S(=O)(O[Na])=O)C8=CC(S(=O)(O[Na])=O)=CC(S(=O)(O[Na])=O)=C78)=O)=CC=C6C)=O)=CC=C5
Clinical Information
– Launched
Research Area
– Cancer; Infection; Cardiovascular Disease
Solubility
– DMSO : 83.33 mg/mL (ultrasonic)|H2O : 50 mg/mL (ultrasonic)
Target
– Apoptosis;Parasite;Phosphatase;Reverse Transcriptase;SARS-CoV;Sirtuin;Topoisomerase
Isoform
– SIRT1;SIRT2;SIRT5
Pathway
– Anti-infection;Apoptosis;Cell Cycle/DNA Damage;Epigenetics;Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






