Download Files:
Sunitinib (Malate)
SKU
HY-10255-100 mg
Category Reference compound
Tags Apoptosis;Autophagy;Cell Cycle/DNA Damage;Protein Tyrosine Kinase/RTK, Apoptosis;Autophagy;IRE1;Mitophagy;PDGFR;VEGFR, Cancer
$60 – $114
Products Details
Product Description
– Sunitinib Malate (SU 11248 Malate) is a multi-targeted receptor tyrosine kinase inhibitor with IC50s of 80 nM and 2 nM for VEGFR2 and PDGFRβ, respectively[1]. Sunitinib Malate, an ATP-competitive inhibitor, effectively inhibits autophosphorylation of Ire1α by inhibiting autophosphorylation and consequent RNase activation[2].
Web ID
– HY-10255
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
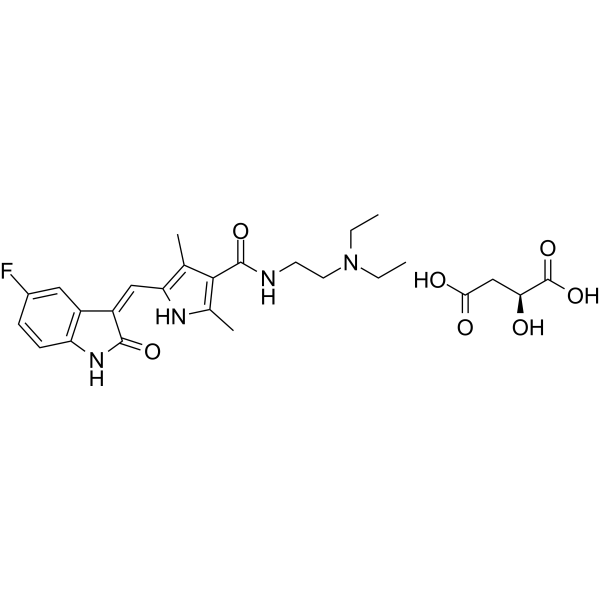
Molecular Formula
– C26H33FN4O7
Citations
– EBioMedicine. 2018 Nov;37:344-355.|Food Chem Toxicol. 2023 Mar 25;113743.|Harvard Medical School LINCS LIBRARY|Oncotarget. 2017 Jul 27;8(56):95116-95134. |University of Michigan. 2021 Jun.|Acta Pharmacol Sin. 2021 Jan;42(1):108-114.|Arch Toxicol. 2019 Jun;93(6):1697-1712. |Biomacromolecules. 2021 Jun 2.|Biotechnol Bioeng. 2021 Sep 3.|Blood. 2019 Oct 17;134(16):1323-1336. |Cancer Lett. 2019 Apr 10;447:105-114.|Cancer Lett. 2021 Oct 6;S0304-3835(21)00513-9.|Cancers (Basel). 2022, 14(16), 3917.|Cell Metab. 2021 Sep 8;S1550-4131(21)00375-2.|Chemosphere. 2022 Sep 7;136354.|EMBO J. 2021 Apr 28;e106771.|Exp Cell Res. 2020 Aug 1;393(1):112054.|Front Pharmacol. 2021 Mar 8;12:644342.|Front Pharmacol. 29 April 2021.|Int Immunopharmacol. 2020 Apr;81:106227.|Int J Clin Exp Pathol. 2015 Apr 1;8(4):3871-81.|J Med Chem. 2016 Sep 22;59(18):8456-72. |J Med Chem. 2019 Jul 11;62(13):6083-6101.|J Pharm Anal. 2023 Apr 19.|J Transl Med. 2023 Jan 9;21(1):9.|Med Chem Res. 2017, 26(9), 2007-2014.|Mol Oncol. 2023 Nov 27.|Nat Biomed Eng. 2018 Aug;2(8):578-588.|Oncotarget. 2017 Nov 15;8(67):111110-111118. |Patent. US20220064117A1.|Research Square Preprint. 2022 Feb.|Research Square Preprint. 2022 May.|Sci Transl Med. 2018 Jul 18;10(450):eaaq1093.|Stem Cell Reports. 2017 Dec 12;9(6):1948-1960.|Ther Adv Med Oncol. 2019 May 17;11:1758835919849757. |Theranostics. 2018 Jul 30;8(15):4262-4278.|Theranostics. 2021 Mar 12;11(11):5387-5403.|Theranostics. 2019 Oct 22;9(26):8377-8391. |Toxicol In Vitro. 2021 Mar;71:105063.|Virulence. 2022 Dec;13(1):1849-1867.
References
– [1]Sun L, et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor r|[2]Ali MM, et al. Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response. EMBO J. 2011 Mar 2;30(5):894-905.|[3]O’Farrell AM, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003 May 1;101(9):3597-605.|[4]Mendel DB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Can
CAS Number
– 341031-54-7
Molecular Weight
– 532.56
Compound Purity
– 99.91
SMILES
– O=C(NCCN(CC)CC)C1=C(NC(/C=C2C(NC3=C2C=C(C=C3)F)=O)=C1C)C.O=C([C@H](CC(O)=O)O)O
Clinical Information
– Launched
Research Area
– Cancer
Solubility
– DMSO : ≥ 15 mg/mL|H2O : 12.5 mg/mL (ultrasonic;adjust pH to 3 with HCl)|H2O : 3.33 mg/mL (ultrasonic;warming;heat to 60°C)
Target
– Apoptosis;Autophagy;IRE1;Mitophagy;PDGFR;VEGFR
Isoform
– PDGFRβ;VEGFR2/KDR/Flk-1
Pathway
– Apoptosis;Autophagy;Cell Cycle/DNA Damage;Protein Tyrosine Kinase/RTK
Product type
– Reference compound
Disclaimer: All products are for research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation.





