Download Files:
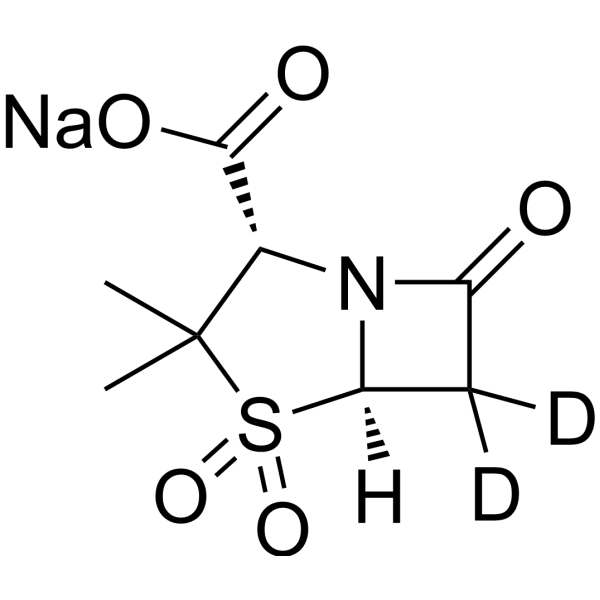
Sulbactam-d2 (sodium)
SKU
HY-B0334AS1-Get quote
Category Isotope-Labeled Compounds
Tags Anti-infection, Antibiotic;Bacterial, Infection
Products Details
Product Description
– Sulbactam-d2 (sodium) is the deuterium labeled Sulbactam sodium[1]. Sulbactam (CP45899) sodium is a competitive, irreversible beta-lactamase inhibitor. Sulbactam sodium shows antimicrobial activity against multidrug-resistant (MDR) acinetobacter calcoaceticus–Acinetobacter baumannii (Acb) complex[2][3].
Web ID
– HY-B0334AS1
Shipping
– Room temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C8H8D2NNaO5S
References
– [1]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019 Feb;53(2):211-216.|[2]Noguchi JK, et al. Sulbactam: a beta-lactamase inhibitor. Clin Pharm. 1988;7(1):37-51.|[3]Lin HS, et al. Sulbactam treatment for pneumonia involving multidrug-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii complex. Infect Dis (Lond). 201547(6):370-378.|[4]Betrosian AP, et al. Ampicillin-sulbactam: an update on the use of parenteral and oral forms in bacterial infections. Expert Opin Drug Metab Toxicol. 20095(9):1099-1112.
CAS Number
– 948027-82-5
Molecular Weight
– 257.24
SMILES
– O=C([C@@H]1N2[C@](C([2H])([2H])C2=O)([H])S(=O)(C1(C)C)=O)O[Na]
Clinical Information
– No Development Reported
Research Area
– Infection
Solubility
– 10 mM in DMSO
Target
– Antibiotic;Bacterial
Pathway
– Anti-infection
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.