Download Files:
Sovesudil
SKU
HY-109191-10 mg
Category Reference compound
Tags Cell Cycle/DNA Damage;Cytoskeleton;Stem Cell/Wnt;TGF-beta/Smad, Others, ROCK
$500 – $850
Products Details
Product Description
– Sovesudil (PHP-201) is a potent, ATP-competitive, locally acting Rho kinase (ROCK) inhibitor with IC50s of 3.7 and 2.3 nM for ROCK-I and ROCK-II, respectively. Sovesudil lowers intraocular pressure (IOP) without inducing hyperemia[1][2].
Web ID
– HY-109191
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C23H22FN3O3
References
– [1]Ha A, et al. Sovesudil (locally acting rho kinase inhibitor) for the treatment of normal-tension glaucoma: the randomized phase II study [published online ahead of print, 2021 Jul 28]. Acta Ophthalmol. 2021;10.1111/aos.14949. |[2]Van de Velde S, et al. AMA0076, a novel, locally acting Rho kinase inhibitor, potently lowers intraocular pressure in New Zealand white rabbits with minimal hyperemia. Invest Ophthalmol Vis Sci. 2014;55(2):1006-1016. Published 2014 Feb 18.
CAS Number
– 1333400-14-8
Molecular Weight
– 407.44
Compound Purity
– 98.31
SMILES
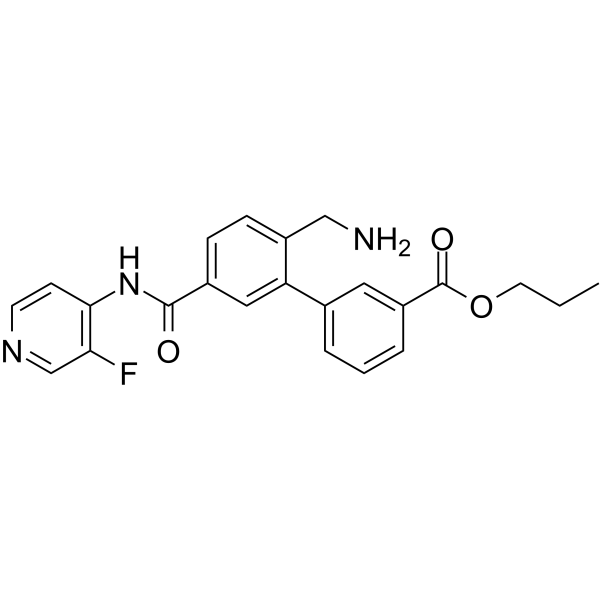
– O=C(C1=CC(C2=CC(C(NC3=C(F)C=NC=C3)=O)=CC=C2CN)=CC=C1)OCCC
Clinical Information
– Phase 3
Research Area
– Others
Solubility
– DMSO : 100 mg/mL (ultrasonic)
Target
– ROCK
Isoform
– ROCK1;ROCK2
Pathway
– Cell Cycle/DNA Damage;Cytoskeleton;Stem Cell/Wnt;TGF-beta/Smad
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






