Download Files:
Sitaxsentan
SKU
HY-76520-Get quote
Category Reference compound
Tags Cardiovascular Disease; Endocrinology, Endothelin Receptor, GPCR/G Protein
Web ID
– HY-76520
Shipping
– Room temperature
Applications
– Metabolism-protein/nucleotide metabolism
Molecular Formula
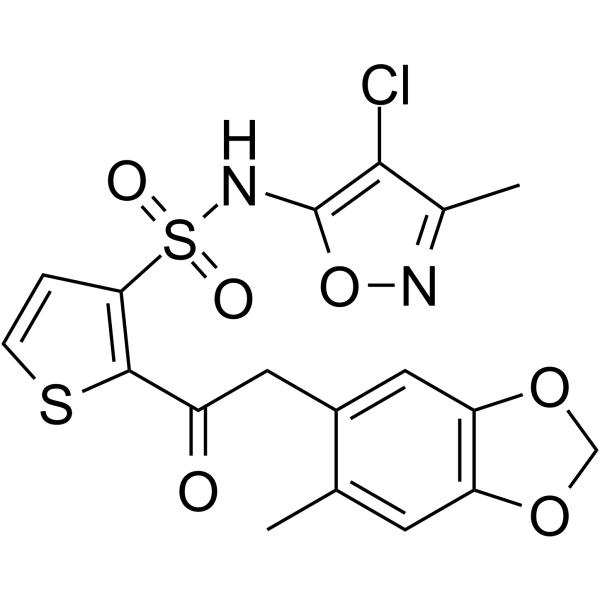
– C18H15ClN2O6S2
References
– [1]Chin M, Levy RD, Yoshida EM, Byrne MF.Sitaxsentan-induced acute severe hepatitis treated with glucocorticoid therapy.Can Respir J. 2012 Jan-Feb;19(1):e1-2.|[2]Sandoval J, et al. STRIDE-4 investigators.Safety and efficacy of sitaxsentan 50 and 100 mg in patients with pulmonary arterial hypertension.Pulm Pharmacol Ther. 2012 Feb;25(1):33-9. Epub 2011 Nov 2.|[3]Safdar Z. Effect of transition from sitaxsentan to ambrisentan in pulmonary arterial hypertension.Vasc Health Risk Manag. 2011;7:119-24. Epub 2011 Mar 2.|[4]Rondelet B, et al. Sildenafil added to sitaxsentan in overcirculation-induced pulmonary arterial hypertension.Am J Physiol Heart Circ Physiol. 2010 Oct;299(4):H1118-23. Epub 2010 Aug 6.|[5]Kaehler, et al. Successful treatment of portopulmonary hypertension with the selective endothelin receptor antagonist Sitaxentan. Wien Klin Wochenschr. 2011 Apr;123(7-8):248-52. Epub 2011 Mar 31.
CAS Number
– 184036-34-8
Molecular Weight
– 454.90
SMILES
– O=S(C1=C(C(CC2=CC3=C(C=C2C)OCO3)=O)SC=C1)(NC4=C(C(C)=NO4)Cl)=O
Clinical Information
– Launched
Research Area
– Cardiovascular Disease; Endocrinology
Solubility
– 10 mM in DMSO
Target
– Endothelin Receptor
Isoform
– ETA
Pathway
– GPCR/G Protein
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






