Download Files:
Sibofimloc
$71 – $850
Products Details
Product Description
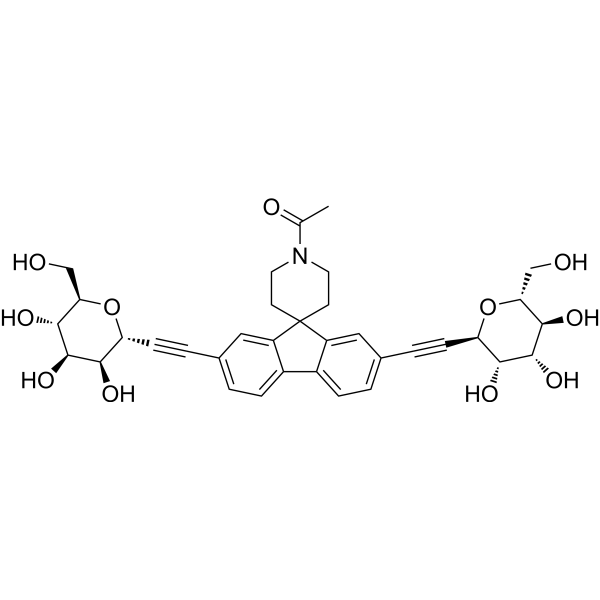
– Sibofimloc (Antibiotic-202) is a first-in-class, gut-restricted, orally active FimH adhesion inhibitor extracted from patent WO2014100158A1, Compound Example 202. Sibofimloc has anti-bacterial infective activity. Sibofimloc is developed for inflammatory bowel disease (IBD)[1][2].
Web ID
– HY-12820
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
– C35H39NO11
References
– [1]W Reinisch, et al. P568 An open-label, multicenter, phase ib, pharmacokinetic (pk) and safety study of a fimh blocker, Sibofimloc (TAK-018/EB8018), in patients with Crohn’s disease (CD), Journal of Crohn’s and Colitis, Volume 14, Issue Supplement_1, January 2020: S479–S480.|[2]Ramtohul, Yeeman K, et al. Preparation of mannose derivatives via coupling and click cycloaddition reactions for treating bacterial infections. From PCT Int. Appl. (2014), WO 2014100158 A1 20140626.
CAS Number
– 1616113-45-1
Molecular Weight
– 649.68
Compound Purity
– 99.63
SMILES
– CC(N(CC1)CCC21C3=CC(C#C[C@@H]4[C@@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O4)=CC=C3C5=CC=C(C#C[C@H]6O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]6O)C=C52)=O
Clinical Information
– No Development Reported
Research Area
– Infection
Solubility
– DMSO : ≥ 31 mg/mL
Target
– Antibiotic;Bacterial
Pathway
– Anti-infection
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






