Download Files:
Saquinavir
SKU
HY-17007-10 mg
Category Reference compound
Tags Anti-infection;Metabolic Enzyme/Protease, HIV;HIV Protease;SARS-CoV, Infection; Cancer
$42 – $396
Products Details
Product Description
– Saquinavir(Ro 31-8959) is an HIV Protease inhibitor used in antiretroviral therapy. Saquinavir is also a SARS-CoV 3CLpro inhibitor with an IC50 of 1.36 μM.
Web ID
– HY-17007
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
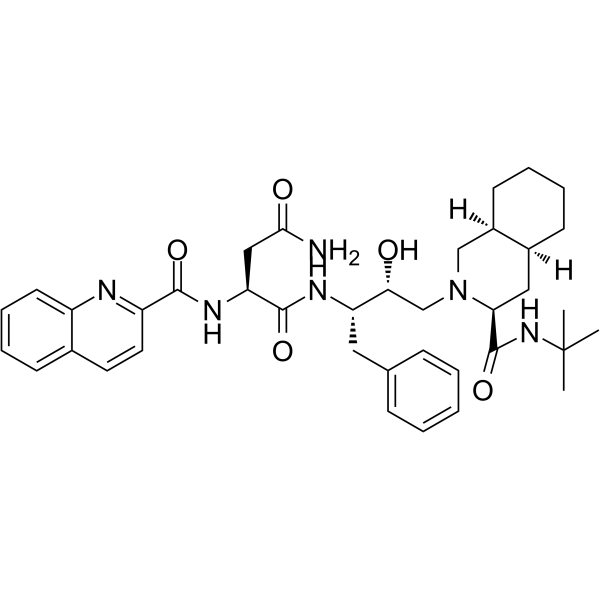
– C38H50N6O5
Citations
– Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00872-20.|Antiviral Res. 2022 Nov 10;105463.|Exp Cell Res. 2019 Oct 1;383(1):111488.|Int J Antimicrob Agents. 2019 Dec;54(6):814-819.|J Med Chem. 2021 Mar 11;64(5):2725-2738.|J Pharm Sci. 2017 Sep;106(9):2839-2846.|Nat Commun. 2020 Sep 4;11(1):4417.|Phytomedicine. 2019 Mar 15;56:175-182. |Signal Transduct Target Ther. 2021 May 29;6(1):212.|bioRxiv. 2020 Apr.
References
– [1]Marco Donia, Danijela Maksimovic-Ivanic, Sanja Mijatovic, et al. In vitro and in vivo anticancer action of Saquinavir-NO, a novel nitric oxide-derivative of the protease inhibitor saquinavir, on hormone resistant prostate cancer cells. Cell cycle. 2011, 10(3): 492 – 499.|[2]Walmsley Sharon, Avihingsanon Anchalee, Slim Jihad et al. Gemini: A Noninferiority Study of Saquinavir/Ritonavir Versus Lopinavir/Ritonavir as Initial HIV-1 Therapy in Adults. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2009,50 (4) :367-374.|[3]Saquinavir|[4]Barillari Giovannia, Iovane Andréa, Bacigalupo Ilariaa, et al. Ritonavir or saquinavir impairs the invasion of cervical intraepithelial neoplasia cells via a reduction of MMP expression and activity. AIDS. 2012, 26 (8): 909-919.|[5]Martha Stefanidou, Carolina Herrera, Naomi Armanasco et al. Saquinavir Inhibits Early Events Associated with Establishment of HIV-1 Infection: Potential Role for Protease Inhibitors in Prevention. Antimicrob. Agents Chemother. 2012, 56 (8): 4381-4390.|[6]Qi Sun, et al. Bardoxolone and bardoxolone methyl, two Nrf2 activators in clinical trials, inhibit SARS-CoV-2 replication and its 3C-like protease. Signal Transduct Target Ther. 2021 May 29;6(1):212.
CAS Number
– 127779-20-8
Molecular Weight
– 670.84
Compound Purity
– 99.73
SMILES
– O=C(N[C@@H](CC(N)=O)C(N[C@@H](CC1=CC=CC=C1)[C@H](O)CN2[C@H](C(NC(C)(C)C)=O)C[C@@](CCCC3)([H])[C@@]3([H])C2)=O)C4=NC5=CC=CC=C5C=C4
Clinical Information
– Launched
Research Area
– Infection; Cancer
Solubility
– DMSO : 100 mg/mL (ultrasonic)
Target
– HIV;HIV Protease;SARS-CoV
Isoform
– HIV-1;HIV-2
Pathway
– Anti-infection;Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





