Download Files:
Rilapladib
SKU
HY-102004-10 mg
Category Reference compound
Tags Inflammation/Immunology; Neurological Disease, Metabolic Enzyme/Protease, Phosphatase
$290 – $2,400
Products Details
Product Description
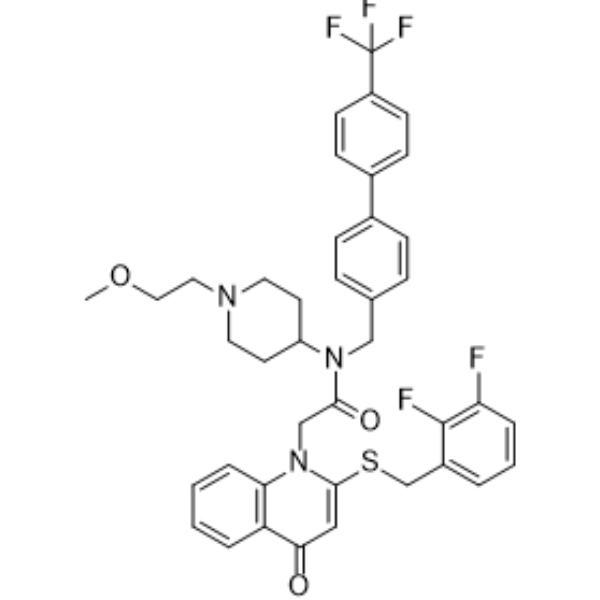
– Rilapladib (SB 659032) is a selective Lp-PLA2 (lipoprotein-associated phospholipase A2) inhibitor with an IC50 of 230 pM[1]. Rilapladib (SB 659032) is also a PAFR (Platelet Activating Factor Receptor) antagonist[2].
Web ID
– HY-102004
Storage Temperature
– -20°C, 3 years (Powder)
Shipping
– Blue Ice
Applications
– Neuroscience-Neuromodulation
Molecular Formula
– C40H38F5N3O3S
References
– [1]Athanasios Papakyriakou, et al. Computational Investigation of Darapladib and Rilapladib Binding to Platelet Activating Factor Receptor. A Possible Mechanism of Their Involvement in Atherosclerosis. International Journal of Chemistry; Vol. 6, No. 1; 2014.|[2]Shaddinger BC, et al. Platelet aggregation unchanged by lipoprotein-associated phospholipase A₂ inhibition: results from an in vitro study and two randomized phase I trials. PLoS One. 2014 Jan 27;9(1):e83094.
CAS Number
– 412950-08-4
Molecular Weight
– 735.81
Compound Purity
– 99.71
SMILES
– O=C(N(C1CCN(CCOC)CC1)CC2=CC=C(C3=CC=C(C(F)(F)F)C=C3)C=C2)CN4C(SCC5=CC=CC(F)=C5F)=CC(C6=C4C=CC=C6)=O
Clinical Information
– Phase 2
Research Area
– Inflammation/Immunology; Neurological Disease
Solubility
– DMSO : 86.67 mg/mL (ultrasonic)
Target
– Phosphatase
Pathway
– Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






