Download Files:
Rezivertinib
SKU
HY-109189-10 mg
Category Reference compound
Tags Cancer, EGFR, JAK/STAT Signaling;Protein Tyrosine Kinase/RTK
$300 – $2,250
Products Details
Product Description
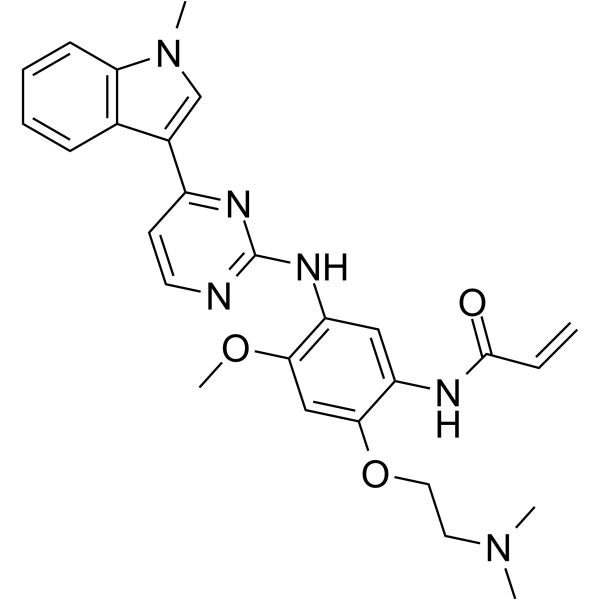
– Rezivertinib (BPI-7711) is an orally active, highly selective and irreversible third-generation EGFR tyrosine kinase inhibitor (TKI). Rezivertinib exhibits high potency against the common activation EGFR and the resistance T790M mutations. Rezivertinib has excellent central nervous system (CNS) penetration and has antitumor activity[1][2].
Web ID
– HY-109189
Storage Temperature
– -20°C, 3 years; 4°C, 2 years (Powder)
Shipping
– Room Temperature
Applications
– Cancer-Kinase/protease
Molecular Formula
– C27H30N6O3
References
– [1]Misako Nagasaka, et al. Beyond Osimertinib: The Development of Third-Generation EGFR Tyrosine Kinase Inhibitors For Advanced EGFR+ NSCLC. J Thorac Oncol. 2021 May;16(5):740-763.|[2]Victoria L. Wilde, et al. Preclinical evidence of BPL-7711 activity in Egfr-mutant non-smallcell lung cancer ( NSCLC ) in orthotopically implanted human tumorxenografts in the lung and brain.
CAS Number
– 1835667-12-3
Molecular Weight
– 486.57
Compound Purity
– 99.43
SMILES
– C=CC(NC1=CC(NC2=NC=CC(C3=CN(C)C4=C3C=CC=C4)=N2)=C(OC)C=C1OCCN(C)C)=O
Clinical Information
– Phase 3
Research Area
– Cancer
Solubility
– DMSO : 125 mg/mL (ultrasonic)
Target
– EGFR
Isoform
– EGFR/ErbB1/HER1
Pathway
– JAK/STAT Signaling;Protein Tyrosine Kinase/RTK
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






