Download Files:
Reparixin (L-lysine salt)
SKU
HY-15252-10 mg
Category Reference compound
Tags Cancer; Inflammation/Immunology; Endocrinology, CXCR, GPCR/G Protein;Immunology/Inflammation
$30 – $384
Products Details
Product Description
– Reparixin L-lysine salt is an allosteric inhibitor of chemokine receptor 1/2 (CXCR1/2) activation.
Web ID
– HY-15252
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– COVID-19-immunoregulation
Molecular Formula
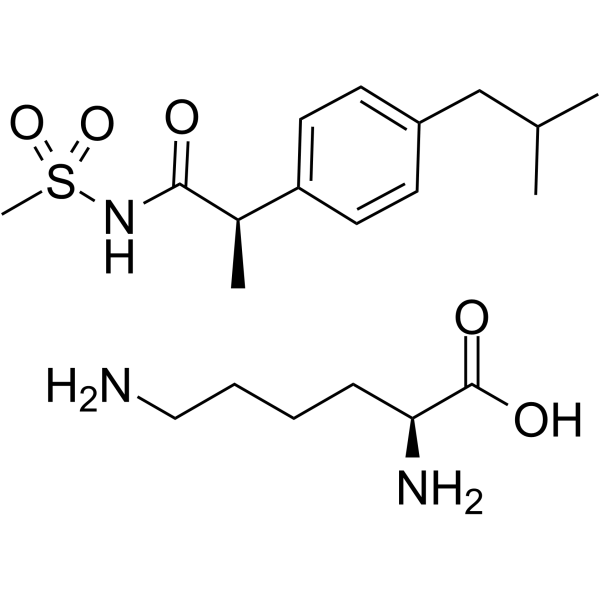
– C20H35N3O5S
Citations
– Ann Rheum Dis. 2016 Apr;75(4):721-9.|Biomed Res Int. 2020 Aug 5;2020:4670604.|bioRxiv. 2020 Sep 12;2020.08.25.265561.|Neurobiol Dis. 29 October 2021, 105538.|Patent. US20170105971A1.|Patent. US20170181987A1.|Ann Rheum Dis. 2016 Apr;75(4):730-8.|Biochim Biophys Acta. 2017 Jan;1863(1):220-230.|BMC Med. 2022 Feb 8;20(1):55.|Cancer Cell Int. 2021 Jul 3;21(1):337.|Cancer Cell. 2023 Apr 10;41(4):693-710.e8.|Cancers (Basel). 2023 Sep 4;15(17):4422.|Cell Biosci. 2019 Jan 3;9:4. |Cell Commun Signal. 2023 Mar 13;21(1):59.|Cell Death Dis. 2017 Jul 13;8(7):e2932.|Cells. 2022 Jun 21;11(13):1986.|Cells. 2022, 11(15), 2402.|EBioMedicine. 2019 May;43:487-500. |FACETS. 2020 Jul.|Front Immunol. 17 October 2022.|Front Immunol. 2021 May 7;12:667177.|Front Immunol. 2018 Sep 12;9:2058.|Gene. 2018 Nov 30;677:149-162.|J Allergy Clin Immunol. 2018 Jun;141(6):2286-2289.e5.|J Cell Mol Med. 2020 Jan;24(2):1588-1598.|J Cell Mol Med. 2020 Nov;24(21):12608-12618.|J Gastroenterol Hepatol. 2018 Feb;33(2):431-442.|J Immunol. 2018 Jul 15;201(2):814-820. |J Mol Cell Biol. 2023 Apr 18;mjad025.|J Mol Med (Berl). 2019 Jan;97(1):25-35. |Johns Hopkins University. Department of Chemical and Biomolecular Engineering. 2016 Dec.|Karolinska Institutet. Dept of Physiology and Pharmacology. 2022 Feb.|Mol Immunol. 2018 Aug 7;101:440-449. |Mol Ther. 2022 Jul 14;S1525-0016(22)00429-4.|Nat Commun. 2017 May 26;8:15584.|Oncogenesis. 2016 Jun 13;5(6):e234. |Oncotarget. 2017 Jul 21;8(36):60210-60222.|Oncotarget. 2018 Aug 24;9(66):32556-32569. |Patent. US20220251222A1.|Research Square Preprint. 2021 Mar.|Research Square Preprint. 2022 Jan.|Sci Adv. 2019 May 8;5(5):eaav7384.|Sci Rep. 2017 Nov 6;7(1):14510. |Stem Cells. 2015 Dec;33(12):3558-68. |University of Michigan. 2018 Oct.|Vet Immunol Immunopathol. 2016 Dec;182:52-58. |Xenotransplantation. 2018 Mar;25(2):e12385.
References
– [1]Moriconi A, et al. Design of noncompetitive interleukin-8 inhibitors acting on CXCR1 and CXCR2. J Med Chem. 2007 Aug 23;50(17):3984-4002.|[2]Midgley I, et al. Species differences in the pharmacokinetics and metabolism of reparixin in rat and dog. Xenobiotica. 2006 May;36(5):419-40|[3]Bertini R, et al. Receptor binding mode and pharmacological characterization of a potent and selective dual CXCR1/CXCR2non-competitive allosteric inhibitor. Br J Pharmacol. 2012 Jan;165(2):436-54.|[4]Catrina, Anca, et al. METHODS AND COMPOUNDS FOR THE TREATMENT OF BONE LOSS AND/OR PAIN. US 20170105971 A1.|[5]Bertini R, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci U S A. 2004 Aug 10;101(32):11791-6.
CAS Number
– 266359-93-7
Molecular Weight
– 429.57
Compound Purity
– 99.93
SMILES
– N[C@@H](CCCCN)C(O)=O.CS(=O)(NC([C@@H](C1=CC=C(CC(C)C)C=C1)C)=O)=O
Clinical Information
– Phase 3
Research Area
– Cancer; Inflammation/Immunology; Endocrinology
Solubility
– DMSO : 100 mg/mL (ultrasonic)|H2O : 100 mg/mL (ultrasonic)
Target
– CXCR
Isoform
– CXCR1;CXCR2
Pathway
– GPCR/G Protein;Immunology/Inflammation
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.





![9-β-D-[2'-Fluoro-2'-deoxy-arabinofuranosyl]-guanin](https://immunomart.com/wp-content/uploads/2024/02/HY-W334680-350x350.gif)

