Download Files:
Rapamycin (GMP)
Products Details
Product Description
– Rapamycin (Sirolimus) (GMP) is Rapamycin (HY-10219) produced by using GMP guidelines. GMP small molecules works appropriately as an auxiliary reagent for cell therapy manufacture. Rapamycin is a potent and specific mTOR inhibitor[1][2][3].
Web ID
– HY-10219G
Shipping
– Room temperature
Molecular Formula
– C51H79NO13
References
– [1]Lee KW, et al. Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells Dev. 2010 Apr;19(4):557-68.|[2]Martin KA, et al. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem. 2007 Dec 7;282(49):36112-20.|[3]Ogawa T, et al. Osteoblastic differentiation is enhanced by rapamycin in rat osteoblast-like osteosarcoma (ROS 17/2.8) cells. Biochem Biophys Res Commun. 1998 Aug 10;249(1):226-30.
CAS Number
– 53123-88-9
Molecular Weight
– 914.17
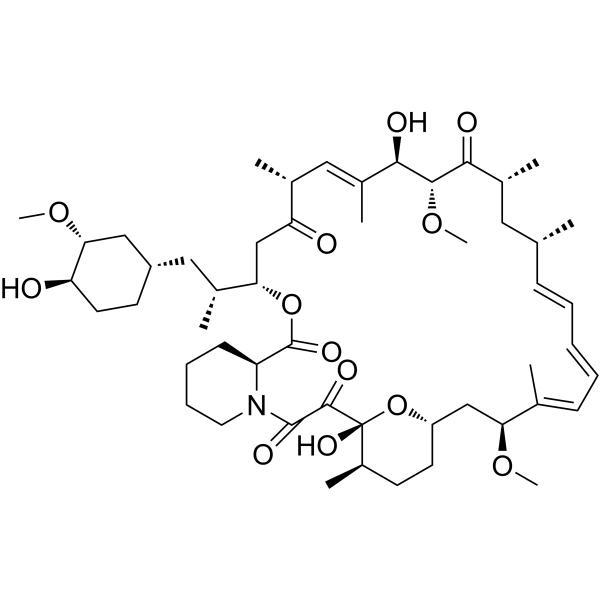
SMILES
– O=C([C@@]1(O)[C@@H](CC[C@@H](C[C@@H](/C(C)=C/C=C/C=C/[C@H](C[C@@H](C)C([C@@H]([C@@H](/C(C)=C/[C@H]2C)O)OC)=O)C)OC)O1)C)C(N3CCCC[C@H]3C(O[C@@H](CC2=O)[C@@H](C[C@H]4C[C@H]([C@H](O)CC4)OC)C)=O)=O
Clinical Information
– Phase 4
Research Area
– Cancer
Solubility
– 10 mM in DMSO
Target
– mTOR
Pathway
– PI3K/Akt/mTOR
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.






