Download Files:
Raltegravir (potassium)
SKU
HY-10353A-10 mg
Category Reference compound
Tags Anti-infection;Metabolic Enzyme/Protease, HIV;HIV Integrase, Infection; Cancer
$30 – $220
Products Details
Product Description
– Raltegravir (MK 0518) potassium is a potent integrase (IN) inhibitor, used to treat HIV infection.
Web ID
– HY-10353A
Storage Temperature
– 4°C (Powder, sealed storage, away from moisture)
Shipping
– Room Temperature
Applications
– COVID-19-anti-virus
Molecular Formula
– C20H20FKN6O5
Citations
– Plant Biosyst. 2018, 1-8.|PLoS One. 2018 Mar 30;13(3):e0195168.|Bioorg Med Chem. 2019 Sep 1;27(17):3836-3845. |Clin Drug Investig. 2019 Mar;39(3):285-299. |J Infect Dis. 2022 Sep 19;jiac386.|J Mol Biol. 2022 Feb 22;167507.|J Neuroimmune Pharmacol. 2017 Dec;12(4):682-692.|J Virol. 2017 Jan 18;91(3). pii: e02152-16.|Life Sci. 9 September 2022, 120948.|Molecules. 2022 Dec 12;27(24):8829.|PeerJ Physical Chemistry. 2019, 1:e6.|Pharmaceuticals. 2023 Aug 8, 16(8), 1118.|Phytomedicine. 2016 Nov 15;23(12):1383-1391. |SSRN. 2023 Mar 30.|Virol Sin. 2023 May 10;S1995-820X(23)00054-8.|Virology. 2023 Jun 21.|Viruses. 2021 Jan 18;13(1):E131.
References
– [1]Moss DM, et al. Divalent metals and pH alter raltegravir disposition in vitro. Antimicrob Agents Chemother. 2012 Jun;56(6):3020-6|[2]Lewis, M.G., et al. Response of a simian immunodeficiency virus (SIVmac251) to raltegravir: a basis for a new treatment for simian AIDS and an animal model for studying lentiviral persistence during antiretroviral therapy. Retrovirology, 2010. 7: p. 21.|[3]Hare S, et al. Structural and functional analyses of the second-generation integrase strand transfer inhibitor dolutegravir (S/GSK1349572). Mol Pharmacol. 2011 Oct;80(4):565-72.|[4]Hare, S., et al., Molecular mechanisms of retroviral integrase inhibition and the evolution of viral resistance. Proc Natl Acad Sci U S A, 2010. 107(46): p. 20057-62.|[5]Hicks C, et al. Raltegravir: the first HIV type 1 integrase inhibitor. Clin Infect Dis. 2009 Apr 1;48(7):931-9
CAS Number
– 871038-72-1
Molecular Weight
– 482.51
Compound Purity
– 99.96
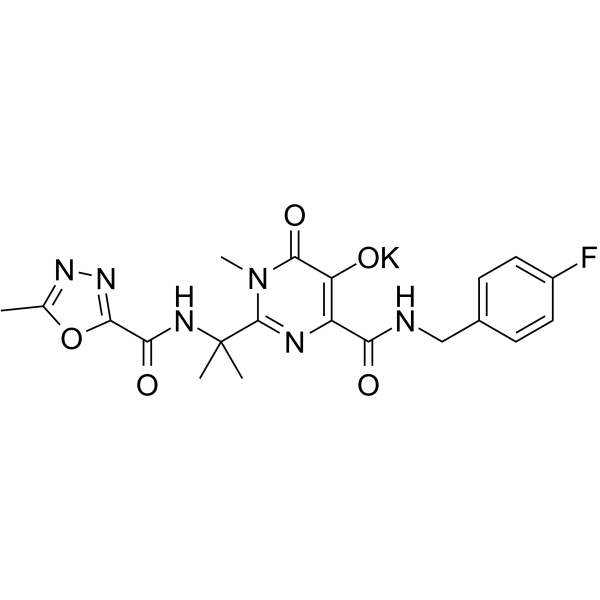
SMILES
– O=C(C(N=C(C(NC(C1=NN=C(C)O1)=O)(C)C)N2C)=C(O[K])C2=O)NCC3=CC=C(F)C=C3
Clinical Information
– Launched
Research Area
– Infection; Cancer
Solubility
– DMSO : 20.83 mg/mL (ultrasonic;warming;heat to 60°C)|H2O : 25 mg/mL (ultrasonic)
Target
– HIV;HIV Integrase
Isoform
– HIV-1;HIV-2
Pathway
– Anti-infection;Metabolic Enzyme/Protease
Product type
– Reference compound
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.




![9-β-D-[2'-Fluoro-2'-deoxy-arabinofuranosyl]-guanin](https://immunomart.com/wp-content/uploads/2024/02/HY-W334680-350x350.gif)


