Download Files:
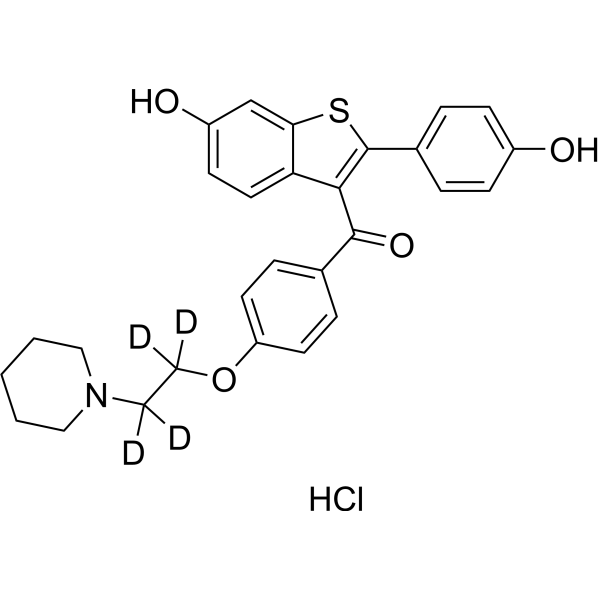
Raloxifene-d4 (hydrochloride)
SKU
HY-13738S2-1 mg
Category Isotope-Labeled Compounds
Tags Cancer, Estrogen Receptor/ERR;Isotope-Labeled Compounds, Others;Vitamin D Related/Nuclear Receptor
Products Details
Product Description
– Raloxifene-d4 (hydrochloride) is the deuterium labeled Raloxifene. Raloxifene (Keoxifene) is a benzothiophene-derived selective estrogen receptor modulator (SERM). Raloxifene has estrogen-agonistic effects on bone and lipids and estrogen-antagonistic effects on the breast and uterus. Raloxifene is used for breast cancer and osteoporosis research[1].
Web ID
– HY-13738S2
Shipping
– Room temperature
Molecular Formula
– C28H24D4ClNO4S
References
– [1]Khovidhunkit W, et al. Clinical effects of raloxifene hydrochloride in women. Ann Intern Med. 1999;130(5):431-439.|[2]Xu H, et al. Effect of caffeine on ovariectomy-induced osteoporosis in rats. Biomed Pharmacother. 2019;112:108650.|[3]Russak EM, et al. Impact of Deuterium Substitution on the Pharmacokinetics of Pharmaceuticals. Ann Pharmacother. 2019;53(2):211-216.
CAS Number
– 1188263-47-9
Molecular Weight
– 514.07
SMILES
– O=C(C1=CC=C(C=C1)OC([2H])([2H])C([2H])([2H])N2CCCCC2)C3=C(C4=CC=C(C=C4)O)SC5=CC(O)=CC=C53.Cl
Clinical Information
– No Development Reported
Research Area
– Cancer
Solubility
– 10 mM in DMSO
Target
– Estrogen Receptor/ERR;Isotope-Labeled Compounds
Pathway
– Others;Vitamin D Related/Nuclear Receptor
Product type
– Isotope-Labeled Compounds
Disclaimer: All products are for Research use only unless clearly stated otherwise on the product datasheet. Datasheets provided on the website are drafts for reference purpose only and you are requested to always refer to the hard copy included in the kit for your experimentation. Agdia Products are available for delivery only in Canada.